Surgical Anatomy
| Primary authors |
|
|---|
Successful surgery requires a thorough knowledge of anatomy. All glaucoma-related procedures require a thorough understanding of the terrain of the external eye, as well as an intimate relationship with the contents of the anterior chamber angle. A discussion of the gross and surgical anatomy of the eye follows, with special considerations for the pediatric patient and patients with other less common conditions.
Gross And Surgical Anatomy
Knowledge of the boundaries and composition of the anterior chamber angle, in addition to the anatomical and surgical limbus, is critical to determine the location and depth of all incisions (including those with lasers) performed during glaucoma procedures. Appreciation of the ocular anatomy that may be affected or complicated during surgery will also allow the surgeon to remedy a potentially dangerous situation in a timely and efficient manner. Our discussion will follow an “inside -out” approach, first reviewing the anatomy of the angle and surrounding structures, then the surgical anatomy of the eye.
Drainage Angle
The drainage angle is responsible for the outflow of aqueous humor from the anterior chamber, and thus provides the primary resistance against this outflow, contributing to and maintaining intraocular pressure (IOP).
Anatomy of the Angle
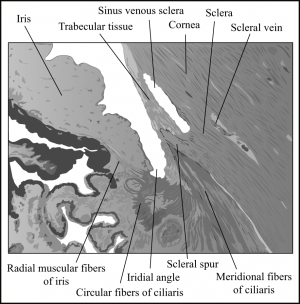
The angle is an anatomically small yet physiologically indispensible region of the eye. The angle is formed by the intersection of the trabecular meshwork and the attachment of the peripheral iris. It contains the anterior extension of the ciliary muscle, the scleral spur, and Schlemm’s canal within its boundaries.
One way to consider the anatomy of this area is to follow the outflow of aqueous humor through it. After its formation by the ciliary processes in the posterior chamber, the aqueous humor flows around the lens and iris, through the pupil, and into the angle of the anterior chamber. Here, in the anterior chamber, the aqueous first encounters the trabecular meshwork.
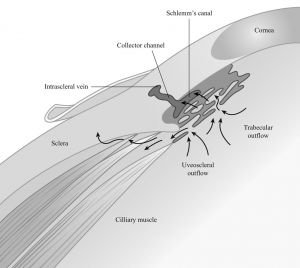
The trabecular meshwork is triangular in cross-section, and its base rests on the scleral spur, with a small region abutting the ciliary muscle (Figure 2-1). Its apex is tethered by the most peripheral region of cornea and the termination of Descemet’s membrane, known as Schwalbe’s line. Aqueous encounters the trabecular meshwork for the first time through the uveal meshwork, a histological subregion of the trabeculum. It proceeds further into the corneoscleral meshwork and, finally, into the juxtacanalicular tissue. The juxtacanalicular tissue is the interface with the inner wall of Schlemm’s canal, a primary area of aqueous fluid resistance in the eye, which establishes physiologic and potentially pathologic IOPs.[1][2][3]
The fluid that traverses the Schlemm’s canal’s inner endothelial wall flows some distance to reach any of the nearly 25 to 35 collector channels emanating from it. From the aqueous humor collector channels, the fluid continues to the aqueous veins, episcleral veins, orbital veins, and ultimately the intracranial cavernous venous sinus. Approximately 90% of aqueous outflow follows this path. The remaining 10% is drained through the uveo-scleral pathway, with the anterior aspect of the ciliary muscle as its interface (Figure 2-2).
Gonioscopic Anatomy of the Angle
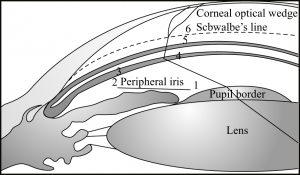
Gonioscopy is the most accurate way of examining angle depth and providing a physiologic perspective for inspection of pathology. Its role in determining angle closure from open-angle glaucoma makes it an essential skill for all ophthalmologists. Gonioscopy also allows the ophthalmologist to appreciate the patient’s anatomy prior to and during procedures, such as laser trabeculoplasty in the adult and goniotomy in the infant. Keeping in mind the anatomy of the angle as described previously, the gonioscopic view makes sense. In a deep view of the angle, with a flat attachment of the peripheral iris, the following structures should be appreciated from anterior to posterior[4] (Figures 2-3 and 2-4):
- Schwalbe’s line: This is the demarcation of the final extent of Descemet’s membrane and its transition toward the unpigmented trabecular meshwork.
- Trabecular meshwork: A fine, granular-appearing structure resembling ground glass is located just posterior to the margin of Schwalbe’s line. The anterior portion of the trabecular meshwork is unpigmented, whereas the posterior portion of the trabecular meshwork is pigmented. The majority of aqueous outflow occurs here.
- Scleral spur: The posterior attachment of the trabecular meshwork. This is the most prominent structure within the angle.
- Ciliary body: The innermost region of the ciliary body is appreciated in the open angle. Located just posterior to the scleral spur, it serves as the aqueous outflow of the uveoscleral pathway.
- Peripheral iris: The insertion of the peripheral iris is best seen when the approach is flat.
Iris
The iris should always be examined and its insertion angle, as viewed by gonioscopy, noted. Rubeosis or other iris abnormalities must be identified, if present, through slit-lamp examination or gonioscopy. Rubeotic irides are prone to bleeding during iridectomy or iris manipulation.[1][2][3]
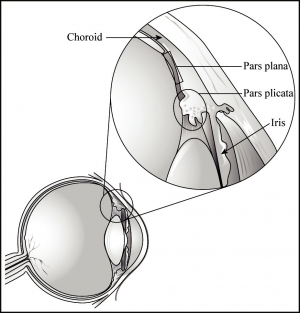
Ciliary Body and Pars Plana
The ciliary body is the most internal of the angle structures. It is composed of the ciliary muscle and the ciliary processes and draws out from the foundation of the iris. At the ora serrata, the flat part of the anterior
ciliary body—the pars plicata—fuses with the choroid. The posterior portion of the ciliary body is the pars plana, the oft-accessed site for performing procedures, such as vitrectomies, injections, and ultrasonic lensectomies. Figure 2-5 shows the relationship of the ciliary body. The outermost fibers of the ciliary muscle attach to the corneoscleral trabecular meshwork and the scleral spur. Approximately 70 ciliary processes project off the pars plicata in a radial fashion. The ciliary processes are found internal to the ciliary muscle and, with the pars plana, form the lateral wall of the posterior chamber. Underlying the iris, the ciliary processes can occasionally be seen gonioscopically with dramatic mydriasis, aniridia, or a surgical coloboma. Otherwise, they are easily visualized endoscopically.[1][2][3] Functionally, the ciliary processes are responsible for the production and inflow regulation of the aqueous humor. For this reason, the ciliary body could be ablated to decrease the production of aqueous humor as a means of IOP reduction in refractory cases. With endocyclophotocoagulation, a laser unit attached to a probe is inserted through a scleral or corneal incision and endoscopically delivers a pulsed, continuous wave to the ciliary processes, thereby causing localized destruction. Transscleral cyclophotocoagulation uses a similar concept but is performed externally. The laser’s focus is delivered 1 to 1.5 mm behind the limbus. It is offset from the aiming beam for the purpose of delivering the maximal amount of energy at the level of the ciliary body.[5]
Surgical Anatomy of the Eye
Limbus
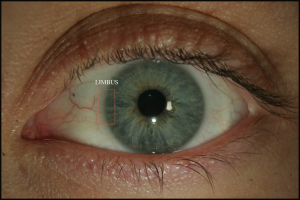
The limbus is one of the most important landmarks in glaucoma surgery. This is the junction between the cornea and the sclera. Grossly, it appears as a faint blue ring around the cornea (Figure 2-6). The clear corneal tissue inserts into white sclera. The anterior portion of the limbus represents the clear cornea, and the posterior end is white scleral tissue. This results in the gray and bluish hue of the limbus. Occasionally, conjunctival vessels can obscure this view; however, a scleral dissection clearly identifies the limbus.
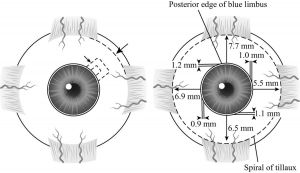
The surgical limbus has a degree of dimensionality, and it is more accurate to conceptualize the limbus as having 2 distinct regions, a border between the cornea and limbus and a border between the sclera and limbus. (Worthen[3] uses the analogy of a “river with two banks.”) The anatomical limbus is defined by its anterior aspect, which overlies a line composed of the ends of Bowman’s layer and Descemet’s membrane. Therefore, the surgical limbus lies anterior to the anatomical limbus. The posterior end overlies the conjunctival tissue, which rests in the iris recess; this is clearly seen as the posterior aspect of the blue limbus. The limbus is much broader in the superior and inferior aspects of the eye as compared to the vertical meridians. The average limbus width is 1.2 mm superiorly, 1.1 mm inferiorly, 1.0 mm nasally, and 0.9 mm temporally (Figure 2-7). For this and other reasons, a superior approach is usually taken in filtration procedures, often in the nasal quadrant, to better allow for future glaucoma or cataract surgery.
The limbus overlies the angle recess. In most cases, a full-thickness perpendicular incision on the scleral edge of the limbus will intersect the
anterior trabecular meshwork. However, variability in the shape of the eye influences its exact orientation and depth. For example, a small or hyperopic eye may have the limbus lying deeper into the angle, whereas a larger or myopic eye will have more depth within the angle recess below and perpendicular to the posterior limbus (Figure 2-8).
Sclera
Over 80% of the globe is covered by sclera. This structure is often surgically manipulated for filtering procedures and traction sutures. Much like the cornea, its main component is type I collagen. As opposed to the cornea, these fibrils are oriented in random fashion, which is the main difference between the optically transparent cornea and the characteristically white sclera. The tendons of the rectus muscles insert into the sclera posterior to the limbus (see Figure 2-7).
The scleral thickness varies by location, ranging from 300 µm to just over 1 mm. The insertions of the rectus muscles are the thinnest areas of the sclera. The sclera is at its thickest around the optic nerve and the posterior pole—approximately 0.86 mm ± 0.26. The sclera thins as it extends anteriorly towards the cornea to the equator (0.42 mm ± 0.14) and ora serrata (0.42 mm ± 0.13). The sclera again thickens slightly at the limbus (0.50 mm ± 0.10). Trabeculectomies are often performed over the area anterior to the equator and directly over the ora serrata. This undoubtedly increases one’s appreciation for the delicate nature of the partial thickness scleral dissection.[6]
Myopes, in particular those who have enlarged axial lengths, have thinner scleras when compared to normal-sized eyes. In comparison to those with normal axial lengths, the sclera is thinner at the equator and posterior pole—a difference of nearly a tenth of a millimeter at the equator and almost three-tenths of a millimeter at the posterior pole.[6]
The sclera is nearly avascular. Two exceptions are the episcleral vessels overlying the sclera, and the intrascleral vascular plexus located posterior to the limbus. Slit-lamp examination will also enable the clinical to identify a number of emissary channels that transmit nerves and vessels.
Conjunctiva
The conjunctiva covers the entirety of the inner eyelid, the fornix, and the external eye up to the limbus. The adequacy of mobility of the conjunctiva should be assessed prior to trabeculectomy or drainage device implantation. Conjunctival hyperemia must always be addressed in the assessment prior to glaucoma surgery. The implications of episcleral vein engorgement or infection are significant, as opposed to irritation from medication or preservative-induced hyperemia.
It is important to appreciate that procedures that violate the conjunctiva may lead to its scarring or reduce its mobility for subsequent procedures. Many times, excessive scarring from prior surgeries will alter the procedure and approach or preclude its utility. Handle the conjunctiva delicately.
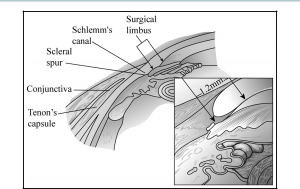
Tenon’s Capsule
Found between the sclera and conjunctiva, Tenon’s capsule is a distinct vascular and elastic connective tissue that arises from the episclera approximately 2 mm posterior to the margin of the cornea and extends across the entire globe to the optic nerve where it fuses with dura mater.
Extraocular Muscles and Their Insertions
Two anterior ciliary arteries mark the insertion of the rectus muscles, with the exception of the lateral rectus muscle, which has one. Underlying the insertion points of the extraocular muscles is the ora serrata internally. A circle can be drawn, with variable distances from the posterior limbus, termed the spiral of Tillaux. The distance of the superior rectus muscle to the posterior limbus is 7.7 mm, 5.5 mm from the medial rectus to the limbus, 6.5 mm at the inferior rectus, and finally 6.9 mm at the lateral rectus (see Figure 2-7).
Considerations of the Surgical Anatomy of the Pediatric Patient
Congenital conditions can cause glaucoma in the infant with distortions of normal anatomy. Apart from pathologic conditions, there are normal developmental anatomic differences of the pediatric patient when compared with the adult. The eye of a newborn is two-thirds the size of an adult’s eye, crowding an already tiny area into a smaller space. Landmarks are not necessarily in their adult configuration until 1 year of age. The following list highlights some significant differences:
- The angle and peripheral iris: The contents of the angle have not fully developed and oriented themselves in their final locations until 6 to 12 months after birth. On gonioscopy, the most characteristic feature of the angle is the trabecular meshwork in the child of age 1 or younger, as opposed to the scleral spur in adults. The peripheral iris attachment is also thinner and flatter in children. In infantile glaucoma, the iris has a more anterior insertion than in a healthy child. Associated with this is a diffuse translucency of the angle structures, creating an obscure view of the ciliary body, trabecular meshwork, and scleral spur. This is known as Barkan’s membrane.
- Ciliary processes: These structures are much thinner and fingerlike in infants and children.
- Limbus: The bluish hue of the limbus is much more difficult to appreciate in the young patient. Other landmarks must then be used. The edge of Bowman’s layer, where the corneal and conjunctival epithelia merge, is one alternative. The texture of the scleral border of the limbus is fragile compared with adults. Procedures in this area must be performed carefully in the pediatric patient for this reason, as it is possible to damage the ciliary body or enter the anterior chamber even with minimal dissection.
- Tenon’s capsule: Tenon’s capsule is more prominent in infants and children compared with adults.
Special Considerations
A number of operations and postoperative conditions exist that alter the external and internal anatomy of the eye, creating a number of unique anatomical considerations. The following discussion is limited to 2 important procedures that have frequent associations with glaucoma.
Prior Vitrectomy and Silicone Oil Injection
Vitrectomy is a common ophthalmic surgery. Secondary glaucomas are a frequent complication of this procedure.[7] It is increasingly important to recognize the anatomical changes and challenges that arise from these procedures. IOP reduction is helpful in the management of these patients.[8] Late effects of silicone oil tamponade can include pupillary block, anterior synechiae, rubeosis iridis, or migration of silicone oil into the angle.[9] In a situation in which the patient is aphakic and has undergone vitrectomy with silicone oil injection, cohesive forces produce a smooth, rounded surface of the silicone bubble. With a peripheral iridectomy and excess silicone oil, the bubble, abutted against the posterior surface of the superior iris (at the 12 o’clock position), will enter the anterior chamber and produce pupillary block. For this reason, a peripheral iridectomy at 6 o’clock can produce an alternate aqueous outflow pathway.[10]
Scleral Buckle
Scleral buckling is another retinal surgery that can dramatically alter the contour and shape of the eye. The procedure has been frequently associated with glaucoma and typical glaucoma visual field deficits. The most common explanation given for this is the external compression-induced IOP elevation. Some argue that the visual field changes following a buckle are in fact due solely to choroidal vascular compression, impeding disc supply. Experimental evidence points toward mechanisms resulting in angle closure. The venous system emptying toward the vortex veins is occluded by the buckle, causing congestion of the ciliary body. As the ciliary body begins
to swell, it pushes the iris anteriorly, resulting in angle closure. Another similar explanation suggests that the obstructed uveoscleral outflow and increased episcleral venous pressure results in elevated IOP.[11]
Because glaucoma can result from or independently develop in an eye operatively treated with a scleral buckle, a filtration or shunting surgery may eventually be needed. Unfortunately, by the very nature of altering the ocular superstructure, excessive crowding and poor conjunctival conditions make tube shunts very difficult to perform procedurally. The anterior chamber tube shunt to an encircling band (ACTEB) procedure developed by Shocket et al[12] has been shown to be efficacious in these cases, as it creates an artificial outflow from the aqueous chamber to an episcleral explant.[12][13]
Conclusion
A thorough understanding of the anatomy related to glaucoma surgery is essential. Familiarity with the anatomy of the angle, limbus, ciliary body, iris, and insertions of the extraocular muscles will enable the surgeon to ably and confidently approach the procedure, and adapt accordingly when necessary. Appreciating the anatomical differences in the pediatric patient dictates the type of procedure performed and can potentially prevent complications. The anatomic changes from scleral buckles, vitrectomies, and other retinal salvage procedures can result in secondary glaucomas. As these complications are responsive to surgical intervention, knowledge of these unique conditions can assist the surgeon greatly.
Key Points
- The angle is composed of the anterior extension of the ciliary muscle, the scleral spur, and Schlemm’s canal.
- The juxtacanalicular tissue is the interface with the inner wall of Schlemm’s canal, the primary area of aqueous fluid resistance.
- Ninety percent of aqueous fluid outflow exits through the trabecular meshwork; the remaining 10% flows through the uveoscleral pathway.
- From anterior to posterior, the gonioscopic appearance of the angle in an adult: Cornea, Schwalbe’s line, nonpigmented trabecular mesh-work, pigmented trabecular meshwork, scleral spur, ciliary body, and peripheral iris.
- The average limbus width is 1.2 mm superiorly, 1.1 mm inferiorly, 1.0 mm nasally and 0.9 mm temporally.
- Tenon’s capsule originates from the episclera 2 mm posterior to the margin of the cornea and extends to the dura mater at the optic nerve.
- The spiral of Tillaux describes the distance of the insertion of the extraocular muscles to the posterior limbus: superior rectus, 7.7 mm; medial rectus, 5.5 mm; inferior rectus, 6.5 mm; and lateral rectus, 6.9 mm.
- The contents of the angle do not orient themselves in their final locations until 6 to 12 months after birth.
- Anatomical changes from surgeries (eg, vitrectomies, silicone oil insertion, and scleral buckles) must be accounted for because they may pose challenges to the treatment of glaucoma.
References
- ↑ 1.0 1.1 1.2 Mills RP, Weinreb RN. Glaucoma Surgical Techniques: Ophthalmology Monographs 4. San Francisco, CA: American Academy of Ophthalmology; 1991.
- ↑ 2.0 2.1 2.2 Trope GE. Glaucoma Surgery. Boca Raton, FL: Taylor & Francis Group; 2005.
- ↑ 3.0 3.1 3.2 3.3 Worthen DM. Anatomical landmarks in glaucoma surgery. Int Ophthalmol Clin. 1981;21(1):15-28.
- ↑ Choplin NT, Lundy DC. Atlas of Glaucoma. 2nd ed. London, UK: Informa UK Limited; 2007.
- ↑ Lin SC. Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. J Glaucoma. 2008;17:238-247.
- ↑ 6.0 6.1 Vurgese S, Panda-Jonas S, Jonas JB. Scleral thickness in human eyes. PLoS ONE. 2012;7(1):e29692.
- ↑ Gedde SJ. Management of glaucoma after retinal detachment surgery. Curr Opin Ophthalmol. 2002;13(2):103-109.
- ↑ Budenz DL, Taba KE, Feuer WJ. Surgical management of secondary glaucoma after pars plana vitrectomy and silicone oil injection for complex retinal detachment. Ophthalmology. 2001;108(9):1628-1632.
- ↑ Honavar SG, Goyal M, Majji AB, et al. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology. 1999;106(1):169-176.
- ↑ Beekhuis WH, Ando F, Zivojnović R, et al. Basal iridectomy at 6 o’clock in the aphakic eye treated with silicone oil: prevention of keratopathy and secondary glaucoma. Br J Ophthalmol. 1987;71(3):197-200.
- ↑ Sato EA, Shinoda K, Inoue M, et al. Reduced choroidal blood flow can induce visual field defect in open angle glaucoma patients without intraocular pressure elevation following encircling scleral buckling. Retina. 2008;28(3):493-497.
- ↑ 12.0 12.1 Schocket SS, Nirankari VS, Lakhanpal V, et al. Anterior chamber tube shunt to an encircling band in the treatment of neovascular glaucoma and other refractory glaucomas. A long-term study. Ophthalmology. 1985;92:553-562.
- ↑ Suh MH, Park KH, Kim TW, Kim DM. The efficacy of a modified ACTSEB (anterior chamber tube shunt to an encircling band) procedure. J Glaucoma. 2007;16(7):622-626.