Glaucoma Surgery in the Nanophthalmic Eye
| Primary authors |
|
|---|
The surgical management of glaucoma in eyes with nanophthalmos requires special consideration because these eyes are prone to serious post-operative complications, including significant inflammation, uveal effusion syndrome, retinal detachment, cystoid maculopathy, aqueous misdirection, and severe vision loss.[1] Fortunately, surgical outcomes have improved due to better recognition of the full clinical spectrum of the “small eye”[2] and advances in cataract surgery, as well as glaucoma surgeries.
Definition and Clinical Features
Nanophthalmos is characterized by a small eye without other malformations. Clinical features include a narrow palpebral fissure, a small orbit, a short axial length, axial hyperopia, a small to normal corneal diameter, a shallow anterior chamber, thickened choroid, thickened sclera, and a high lens-eye volume ratio.[2] The angle-closure form of glaucoma occurs frequently in such eyes. The “pushing” pupillary block mechanism is caused by a relatively large lens in a small anterior segment, which leads to narrowing of the angle and peripheral anterior synechiae (PAS) formation. Another, less common mechanism occurs in the presence of an annular ciliochoroidal effusion in which the anteriorly rotated ciliary processes displace the peripheral iris to close the anterior chamber angle.[3]
Diagnostic evaluation should include refraction, gonioscopy, and biometry measurements of the axial length, keratometry readings, and anterior chamber (AC) depth. Imaging with standard B-scan ultrasound provides helpful information about the combined retinal-choroidal-scleral thickness.[2] Although there are no absolute biometric parameters for nanophthalmos, an axial length <21.0 mm and scleral thickness >1.7 mm support the diagnosis of nanophthalmos.[2] A variety of retinal pathology has been described in these eyes, such as macular hypoplasia, retinal cysts, macular folds, retinoschisis, pigmentary retinopathy, and disc drusen.[2]
Medical and Laser Treatment
Traditionally, the first-line treatment of glaucoma in nanophthalmic eyes has been medical and laser therapy due to the high rate of complications with incisional surgery. Typically, aqueous suppressants such as beta-blockers, alpha-adrenergic agonists, and carbonic anhydrase inhibitors are used. In addition, the prostaglandin outflow agents can also be used to lower intraocular pressure (IOP). Miotic agents should be used with caution, as they may worsen pupillary block by relaxing the zonules, which allows the lens to “push” forward and further crowd the anterior segment.[3][4]
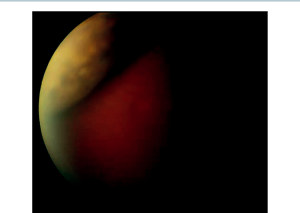
Laser iridotomy can relieve the pupillary block, and iridoplasty can further open the drainage angle.[1][4] These interventions should be per-formed before extensive PAS develops.[4] It is important to remember that nanophthalmic eyes are more prone to significant postoperative inflammation, therefore steroids should be used judiciously. Such patients may develop uveal effusions after laser procedures (Figure 33-1), which may require oral prednisone in addition to topical prednisolone. If such effusions do not respond to aggressive steroid use, unsutured sclerotomies may be required to allow for posterior drainage of the effusions.[4]
Incisional Surgery
The decision for incisional surgery in an eye with nanophthalmos requires careful planning of anesthesia and surgical approach. In such eyes, it is advised to keep a simple approach given the increased risk of complications. As there are no clinical trial results to guide treatments in such eyes, the following considerations are based on clinical experience.
Preoperative Management
If a patient has shown increased propensity for inflammation, such as spontaneous uveal effusions or marked inflammatory reaction after laser treatments, then preoperative prednisone should be considered 2 to 3 days before surgery at a dose of 40 to 60 mg. To minimize the risk of increased posterior pressure and intraoperative aqueous misdirection, mannitol (12.5 to 25 g intravenously) or acetazolamide may be given.
Anesthesia
When planning anesthesia for these patients, it is important to consider general anesthesia. If a patient has a shallow orbit, then an anesthetic block may cause significant posterior pressure and severe chemosis to a degree that may preclude the surgery from being performed.[2] Topical anesthetic surgery is not advised due to the risk of intraoperative aqueous misdirection. If a block is performed, then only a small volume (ie, <3 cc) should be given, and one should consider applying a Honan balloon (The Lebanon Corporation, Lebanon, Indiana) for at least 10 minutes to ensure a soft eye.[5]
Phacoemulsification
Several recent case series have indicated that small-incision phacoemul-sification can be safe and effective in patients with nanophthalmos for both visual improvement and IOP control.[2][5][6] In eyes with no preoperative evidence of choroidal effusion, prophylactic sclerotomies were not necessary, and no postoperative effusions were reported.[2]
Factors to keep in mind when performing phacoemulsification in nanophthalmic eyes include the following:
- Given very shallow ACs with limited working space, great care must be taken to avoid damage to the corneal endothelium. Frequent reapplication of viscoelastic against the endothelium and performing phacoemulsification in-the-bag are important.
- Poorly dilating pupils with posterior synechiae require synechialysis, pupil stretching, and iris retraction, including an iris retractor posterior to the temporal clear cornea incision. Such manipulations can cause significant postoperative inflammation.
- Hydrodissection should be performed carefully to prevent overfill in the capsular bag and iris prolapse through the corneal incision.
- Use increased height of infusion to maintain AC depth, but be aware of potential risk for diverting the infusion into the posterior segment.
- The intraocular lenses (IOLs) required are high-powered and will often need special ordering. If using a single-piece IOL, consider the use of a capsule tension ring, which will provide stable tension at the equator of the residual capsule bag with the implication of a stable planar configuration.
- Consider using a 3-piece IOL because the flexible haptics may provide more capsular tension, help stabilize the zonules, and decrease the risk of aqueous misdirection.
- We do not advise piggyback IOLs (ie, one in-the-bag and one in the sulcus) due to the limited space in the eye and to minimize the risk of the sulcus-placed IOL causing chaffing of the iris and/or ciliary body in eyes that are susceptible to inflammation.
Trabeculectomy
If the IOP is still uncontrolled after lens extraction, a good next option is trabeculectomy. In nanophthalmos, the sudden decrease in IOP on open-ing the globe can result in rapid uveal effusion, secondary retinal detach-ment, vitreous hemorrhage, and aqueous misdirection.[3][6] Several surgical modifications to trabeculectomy have been suggested to minimize the risk of these complications, including the following[3]:
- Stabilizing the anterior chamber depth with viscoelastic prior to performing the sclerostomy.
- Pre-placing the scleral flap sutures.
- Tight scleral flap closure.
- Leaving viscoelastic in the AC.
- Inferior sclerotomies or sclerectomies.
To decrease the risk of trabeculectomy failure because of the thick sclera, a deep sclerectomy should be considered. After dissecting the scleral flap just anterior to the surgical limbus, the underlying scleral tissue can be dissected off, leaving a thin layer of sclera over the uvea.
Glaucoma Drainage Devices
To our knowledge, there are no studies reporting on surgical results of glaucoma drainage devices in patients with nanophthalmos. Factors to consider are a small orbit and small globe, which may make placement of the plate challenging. Also, there may be limited space in the crowded AC for a tube.
Postoperative Management
It is important to remember that patients with nanophthalmos are prone to significant postoperative inflammation.[2] Frequent topical steroid administration should be a mainstay of postoperative treatment. consideration should also be given to subconjunctival or oral steroids to supplement control of inflammation. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may also be helpful. Other postoperative complications should be managed as with any other case.
Special Considerations in Nanophthalmos
It is of utmost importance to recognize aqueous misdirection and uveal effusions in nanophthalmic eyes. The following management steps are options when these complications occur during specific procedures or dur-ing the postoperative period.
Aqueous Misdirection
When performing any type of incisional surgery on nanophthalmic eyes, there is an increased risk of intraoperative aqueous misdirection, which is characterized by elevated IOP and persistent AC shallowing, with or without accompanying iris prolapse. As noted above in the preoperative management section, mannitol or acetazolamide may help decrease the risk of aqueous misdirection by decreasing vitreous pressure and lowering IOP, respectively.
If intraoperative aqueous misdirection occurs with AC shallowing, the following steps may be taken in the given clinical setting:
During phacoemulsification:
- Administer the hyperosmotic agent, mannitol, intravenously (suggested 12.5 to 25 g single dose, infused slowly over 30 to 60 minutes, but up to 1 to 2 g/kg body weight may be given; however, in the authors’ opinion this large dose is not necessary to achieve the hyperosmotic effect), and aqueous suppressant acetazolamide (500 mg intravenously).
- Raise the bottle height to increase the infusion pressure, but be cautious to avoid introducing more fluid into the vitreous cavity.
- Decrease the aspiration rate, but be cautious to recognize the risk of introducing more fluid into the vitreous cavity.
- Perform a vitrectomy with the goal to disrupt the anterior hyaloid vitreous face—either via a posterior pars plana approach or an anterior approach with iridectomy and hyaloid-zonulectomy. If the IOL has not yet been placed, then a posterior capsulectomy and anterior vitrectomy may be considered.
- Use cycloplegics and aqueous suppressants at the end of the surgery.
- Avoid miotic agents, as this drug class exacerbates aqueous misdirection.
During trabeculectomy:
- Administer the hyperosmotic agent, mannitol, intravenously (see suggested dose in the section above), and aqueous suppressant acetazolamide (500 mg intravenously).
- Perform vitrectomy either through the pars plana approach or an anterior approach through an iridectomy and hyaloid-zonulectomy.
- Use cycloplegics and aqueous suppressants at the end of the surgery.
- Avoid miotic agents, as this drug class exacerbates aqueous misdirection.
During postoperative period:
- Avoid miotic agents, as this drug class exacerbates aqueous misdirection.
- Adminster aqueous suppressants.
- Administer cycloplegics.
- Perform Nd:YAG laser anterior hyaloidotomy.
- Perform surgical pars plana vitrectomy with anterior hyaloidotomy.
Uveal Effusion
In patients with a history of choroidal effusion or an existing choroidal effusion, it may be prudent to perform an unsutured sclerotomy or sclerectomy either several weeks prior to or at the time of planned incisional surgery. This leaves a thinner scleral area open for posterior uveoscleral drainage from the choroid which helps resolve an existing choroidal effusion. Several techniques have been described. Two techniques described by Jin and Anderson,[4] which were successful in a series of patients are as follows:
- Sclerectomy: Remove a partial (two-thirds) thickness rectangle of sclera approximately 5 × 7 mm in a quadrant, then remove a 1-mm, full-thickness piece in the bed.
- Unsutured sclerotomy: Create a full-thickness V-shaped incision through the sclera into the suprachoroidal space. The site should be positioned anteriorly over the pars plana region.
The unsutured sclerotomy technique is preferred because it causes less posterior scleral weakness, which is a risk for rupture with retina and vitreous incarceration. The anterior site is protected by a thicker uveal layer and a firm vitreous base. These procedures may also be performed in the event of postoperative uveal effusion, and the effusion will likely resolve within 2 weeks.
Key Points
- Nanophthalmos is characterized by a small eye with short axial length, shallow anterior chamber, normal lens, thickened choroid and sclera, and a high risk for angle-closure glaucoma.
- Nanophthalmic eyes are prone to significant postoperative inflammation, uveal effusions, and aqueous misdirection, even following uncomplicated laser procedures.
- First-line management of glaucoma should be medical and laser treatment due to increased risk of intra- and postoperative complications.
- Small incision phacoemulsification can often be performed safely and successfully without the need for prophylactic sclerotomies.
- Trabeculectomy can be performed with several minor surgical modifications to minimize the risk of intraoperative uveal effusion and aqueous misdirection.
- It is important to promptly recognize and treat aqueous misdirection and uveal effusion.
References
- ↑ 1.0 1.1 Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982;89(9):1006-1012.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Wu W, Dawson DG, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004;30(3):584-590.
- ↑ 3.0 3.1 3.2 3.3 Yalvac IS, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye (Lond). 2008;22(6):838-843.
- ↑ 4.0 4.1 4.2 4.3 4.4 Jin JC, Anderson DR. Laser and unsutured sclerotomy in nanophthalmos. Am J Ophthalmol. 1990;109(5):575-580.
- ↑ 5.0 5.1 Faucher A, Hasanee K, Rootman DS. Phacoemulsification and intraocular lens implantation in nanophthalmic eyes: report of a medium-size series. J Cataract Refract Surg. 2002;28(5):837-842.
- ↑ 6.0 6.1 Yuzbasioglu E, Artunay O, Agachan A, Bilen H. Phacoemulsification in patients with nanophthalmos. Can J Ophthalmol. 2009;44(5):534-539.