Introduction to Glaucoma
| Primary authors |
|
|---|
This section will include basic information on the diagnosis, pathophysiology and treatment of various forms of glaucoma. Educational material will be added regularly. In many cases, each section will have a video, video transcript as well as added information in the form of figures and tables. Please stop by over the next few weeks for updates.
What is Glaucoma?
https://www.youtube.com/watch?v=Whl_ahkujZ4&t=120s
Edited Video Transcript:
Welcome to a new series called “(1)Slide (5)Minutes” where we will present no more than one content slide and will always keep it at 5 minutes or less. The first question to start with is maybe a bit too ambitious, “What is Glaucoma?” The answer is intended to be appropriate for patients, medical students and residents.
Glaucoma is a general or “umbrella” term that we use to cover a group of diseases that share the common clinical picture of characteristic optic nerve “cupping” with distinctive patterns of visual field loss. The optic nerve ”cupping” is an expansion of the internal optic nerve head void that occurs secondary to the loss of ganglion cell axons (think of them as straws that extend from cells on the retina) that leave the eye through the opening in the sclera in the back of the eye. We diagnose glaucoma though optic nerve head exam looking at the size of the cup and width of the neuroretinal rim as well as other features of the optic nerve (see https://www.youtube.com/watch?v=LlwRgbzRo8U). We also utilize optical coherence tomography (or OCT) to objectively measure the thickness of the retinal nerve fiber layer and other features of the back of the eye including OCT macula scans and, more recently, OCT-Angiography (OCT-A). We use visual field testing to look for the distinct visual field defects that are characteristic of glaucoma (including nasal steps (with superior nasal steps most common, arcuate scotomas and, unfortunately, in some cases paracentral and central scotomas. (see Visual Field Testing). It should be noted that the findings between ONH exam, HVF and OCT are frequently asynchronous and HVF deficits may lag findings on ONH exam and RNFL OCT in which case we refer to the stage of disease as “pre-perimetric” glaucoma meaning it has not manifested on the more subjective HVF perimetry but can be identified objectively by examination.
In the distant past, glaucoma was thought to be a disease of elevated pressure in the eye (or intraocular pressure-IOP), however, we now know that glaucoma can happen at what was thought to be “normal” pressure (10-21mmHg) and we no longer use IOP as part of our definition of glaucoma. Still, IOP is an important risk factor for glaucoma and is the only modifiable risk factor that we can do something about. We treat glaucoma with drops that decrease fluid production (beta blockers and carbonic anhydrase inhibitors) or increase outflow of fluid from the eye (like the prostaglandin analogs and rho kinase inhibitors). Our goal with treatment is to decrease pressure by 25-30% from baseline as a simple rule. We can also use oral medications (like acetazolamide and methazolamide) to decrease IOP. Lasers can be used to increase outflow (laser trabeculoplasty-SLT) or decrease inflow (like cyclophotocoagulation or CPC and the more recent micropulse CPC). Finally, we can use various surgical approaches to decrease pressure when needed (see www.KEOGT.com for a full discussion of surgical interventions).
When measuring IOP, it is important to take diurnal (day time) and nocturnal (night time) fluctuations into account. Pressure is highest in the early morning hours and many patients might have higher pressures outside of normal office hours (making us believe the IOP is within normal range when it often is not). There are other factors that contribute to glaucomatous optic neuropathy (for example age, ethnicity, myopia, genetics, family history) that should be taken into account. Research continues to explore the effects of IOP independent factors such as immunologic causes as well as ocular blood flow connections to advancing disease (despite what appears to be well controlled IOP) all of which remain poorly characterized to date. In summary, glaucoma is a disease of the optic nerve that results in loss of ganglion cells and thinning of the optic nerve neuroretinal rim (producing cupping). The nerve damage leads to characteristic patterns of visual field loss usually sparing the central visual field until advanced disease stages. Treatment is focused on lowering IOP but many other “pressure independent factors” are at play and we continue to research other ways to preserve vision with potential for both neuroprotection as well as aspirational hopes to reverse diseased nerves in the future. Thank you for your time.
Epidemiological Aspects of Glaucoma
VIDEO: https://youtu.be/88HlLaBN6h8
Primary Open Angle Glaucoma
•1.86% of US population over age of 40
•~3.36million people in 2020
•Second leading cause of blindness worldwide (after cataracts)

•Prevalence in patients in their 70s is 3-8x higher than patients in their 40s
•Prevalence is higher in specific populations:
•Black/Latino individuals 4x that of White individuals
•Black patients (age 46-65) with POAG are 15x more likely to be blind compared to white patients of the same age
•Incidence ranges from a 5-year incidence of 1.1% in Australia to 0.72% 5-year incidence in Olmsted County, Minnesota and uniformly increases with age
•Risk Factors: Age, Elevated IOP, Family History, Race/Ethnicity, Corneal Thickness, Perfusion Pressure
- Disputed/Evolving Risk Factors: DM, HTN, IOP Fluctuations and Peak IOP
Primary Angle-Closure Glaucoma
•Prevalence in white population is ~0.1% and 20-40x higher in Inuit population
•PACG in Asian Population is between that of White and Inuit population
•0.46% in Middle Eastern Population to 1.19% in Japanese Population
•0.5% in rural Chinese population over age 40 (wide range depending on study)
•PACG in Black population thought to be similar to white population
•Most common between age of 55 and 65 but reported in children
•Risk Factors: Female, Older Age, Hyperopia with shallow AC
References:
•Schoff EO, Hattenhauer MG, Ing HH, et al. Estimated incidence of open-angle glaucoma in Olmsted County, Minnesota. Ophthalmology. 2001;108(5):882–886.
•Cheng JW, Zong Y, Zeng YY, Wei RL. The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PLoS One. 2014;9(7):e103222. Published 2014 Jul 24.
•Yuanbo Liang, David S. Friedman, Qiang Zhou, Xiao Hui Yang, Lan Ping Sun, Lixia Guo, Dolly S. Chang, Liying Lian, Ning Li Wang, for the Handan Eye Study Group; Prevalence and Characteristics of Primary Angle-Closure Diseases in a Rural Adult Chinese Population: The Handan Eye Study. Invest. Ophthalmol. Vis. Sci. 2011;52(12):8672-8679.
The 5Rs of Examining the Optic Disc:
This lectures takes you through the steps to learning and implementing the optic disc exam https://www.youtube.com/watch?v=LlwRgbzRo8U
Edited Transcript
While I was a resident at the University of Colorado, Allergan put out a series of educational materials that I found to be invaluable for foundational knowledge on the topic of glaucoma. Specifically, the FORGE I program was designed by a panel of experts and then spread across the nation and the world using a series of in person and broadcast lectures. I will cover FORGE II, which focused on visual testing, at a later date…but I thought this was a good time to discuss the basics of optic nerve head evaluation since we have new incoming residents who are likely eager to learn the nuances of examining the optic nerve. Glaucoma is an optic neuropathy that has very characteristic visual field and optic nerve changes. There are multiple risk factors. You can see progressive injury to the retinal ganglion cells and their axons. There is a very specific pattern of optic atrophy, which we refer to as cupping. And there are associated visual functional deficits.
Glaucoma prevalence is increasing as the population ages and it affects more than 4 million individuals over the age of 40 in the United States. Assessment and documentation of the optic nerve and visual field are an essential component to the diagnosis, staging and longitudinal assessment of glaucoma.
Studies have shown that glaucoma patients have trouble with daily activities that involve light and dark adaptation, so going from light and dark into different rooms, glare and loss of peripheral vision in glaucoma, which is believed to increase the likelihood of falls in elderly patients. Independent of visual acuity changes, the loss of visual field was associated with a higher risk of falls as well as with other negative effects on patient’s lives, such as decreased enjoyment of reading and watching television. Other common problems are reading, recognizing faces, going up and down stairs, picking up dropped objects, crossing a road - all things that are very important in daily life.
The Five Rules:
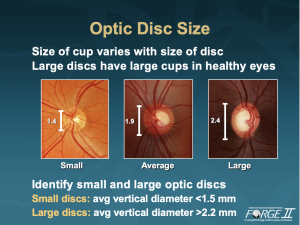
The first rule: First observe the scleral ring to identify the limits of the optic disc and its size. And in brief, we emphasized it with the first rule, the measurement of disc diameter, and in particular, vertical disc diameter. Most commonly and most effectively, measurement of the scleral ring is done in clinical practice at the slit-lamp with the use of a handheld lens and slit-lamp biomicroscopy. Using the 78D lens must be multiplied by 1.1 to yield an absolute measure of the vertical disc diameter. For those who use a 90D lens, multiply the number by 1.3. For those who use a 60D lens, this is a direct measurement and there is no correction factor. The size of the cup varies with the size of the disc. Large discs have large cups in healthy eyes. Here we have three different size healthy optic discs. On the left we are looking at a small diameter optic disc, 1.4 mm. On the right we are looking at a large diameter or macrodisc with a vertical disc diameter of 2.4 mm. In general, smaller microdiscs have an average vertical diameter of less than 1.5 mm. And the larger macrodisc has a vertical diameter of greater than 2.2 mm. Large discs have large cups in healthy eyes.
The second rule is to identify the size of the neuroretinal rim. Measurement of the retinal rim is made using the ISNT Rule, which is ascertained by looking at the rim width, the distance between the border of the disc and the position of the blood vessels bending. The ISNT Rule suggests that in healthy eyes Inferior rim is always wider than the Superior rim, which is wider than the Nasal rim, which is wider than the Temporal rim. In clinical practice it is largely reliable, but it is not perfect. Jeff Liebmann and his colleagues published an article in The Archives of Ophthalmology where they studied healthy and glaucomatous eyes and showed that in about 2/3 of the cases normal eyes obey the ISNT Rule, and that the ISNT Rule is very often not obeyed in eyes with glaucoma.
The third rule is to examine the retinal nerve fiber layer, which is always challenging. Again, in clinical practice you can get a good estimate of the quality of the retinal nerve fiber layer at the slit-lamp using the hand held lens. Typically, when examining the retinal nerve fiber layer, clinicians should look for a characteristic reflection pattern in the superior temporal region where the nerve fiber layer is thickest and the inferotemporal where it is also thickest there are bright reflections reflecting the thick retinal nerve fiber layer. In contrast to the papillomacular region where the retinal nerve fiber layer is rather thin, the reflections are poor and it is dark. When looking at the optic disc, focus on the parapapillary retina to estimate the quality of the retinal nerve fiber layer, and look for this bright, dark, bright pattern of reflections as you move from superotemporal to papillomacula to inferotemporal regions.
In an eye with diffuse retinal nerve fiber loss, the margins of the blood vessels are very sharp because they are imbedded within the retinal nerve fiber layer. The loss of the striation pattern will also be evident. This accounts for about 50% of the patients who develop glaucoma with ocular hypertension. The other patients typically have a focal or local pattern of retinal nerve fiber loss. Most commonly this will occur in the inferotemporal region, followed in terms of frequency by the superotemporal region. This is detectable at the slit-lamp using a handheld lens in color, but a red free or green light sometimes provides a better view. Stereo disc photographs should be a part of the baseline examination for all patients. When the patient returns for follow up, one area to pay particular attention to is the retinal nerve fiber layer, which should be compared with the baseline photographs. Few studies have compared the sensitivity of optic disc and RNFL assessment. From annual examinations of 813 ocular hypertensive eyes, optic disc and nerve fiber layer photographs were compared in 2 age-matched subgroups: 37 eyes that converted to abnormal visual field tests at the end of a 5-year period and 37 control eyes that retained normal field tests. Disc change was detected in only 7 of 37 (19%) converters to field loss and in 1 of 37 (3%) controls. Progressive nerve fiber layer loss was observed in 18 of 37 (49%) converters and in 3 of 37 (8%) controls. In this important study, serial nerve fiber layer examination was more sensitive than color disc photograph evaluation in the detection of progressive glaucoma damage at this early stage of glaucoma.
The fourth rule is examine the region of parapapillary atrophy, the region adjacent to the optic disc. There are two types of parapapillary atrophy: alpha zone and beta zone. Alpha zone is typically more peripheral and it consists of hypo- and hyperpigmented areas. It is present in normal as well as glaucomatous eyes. The beta zone is characteristically adjacent to the optic disc. It consists of atrophy of the retinal pigment epithelium and choriocapillaris. Generally, the large choroidal vessels are visible, especially in glaucomatous eyes. The width of the beta zone inversely correlates with the rim width at the same area. As the patient’s neuroretinal rim thins, typically the beta zone or parapapillary atrophy increases in size.
The fifth rule is to look for retinal and optic disc hemorrhages. These can be very challenging at times to identify, even for experts. They may be confused with blood vessels, can be either prominent or subtle. When examining baseline stereo photographs at follow-up exams, one of the areas to focus on is whether or not an optic disc hemorrhage is present that might have been missed during the clinical examination.
Example of 5 rules to determine if a patient has glaucoma
1 – Disc size – This patient has a small disc
2 – ISNT Rule - The ISNT Rule is not obeyed. The inferior rim is narrower than the superior rim. This should be a red flag suggesting that the patient may have glaucoma.
3 – RNFL Defects - Looking carefully at the retinal nerve fiber layer there is a wedge-shaped retinal nerve fiber layer defect.
4 – Parapillary atrophy - There is no significant parapapillary atrophy in this patient.
5 – Hemorrhage - There is a minute parapapillary optic disc hemorrhage.
Does this patient have glaucoma? Yes
References
Colon-Emeric CS, Biggs DP, Schenck AP, Lyles KW. Risk factors for hip fracture in skilled nursing facilities: who should be evaluated? Osteoporos Int. 2003;14:484-489.
Guse CE, Porinsky R. Risk factors associated with hospitalization for unintentional falls: Wisconsin hospital discharge data for patients aged 65 and over. WMJ. 2003;102:37-42.
McGwin G, Jr., Owsley C, Ball K. Identifying crash involvement among older drivers: agreement between self-report and state records. Accid Anal Prev. 1998;30:781-791.
Harizman N, Oliveira C, Chiang A, Tello C, Marmor M, Ritch R, Liebmann JM. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006 Nov;124(11):1579-1583.
