Lens Induced Glaucoma
| Primary authors |
|
|---|
Lens induced glaucoma can be subdivided into distinct categories that include: 1. Phacolytic Glaucoma, 2. Lens Particle Glaucoma and 3. Phacoantigenic Glaucoma.
Phacolytic Glaucoma
WATCH LECTURE HERE: https://youtu.be/M5UwSS7BcG0
Phacolytic glaucoma is a form of secondary open angle glaucoma that is related to
leakage of high molecular weight proteins through microscopic openings in the capsule of a hypermature cataract. This leads to inflammation with clogging of the trabecular meshwork by proteins as well as macrophages that engulf the proteins and other inflammatory debris. All of these factors lead to an increase in intraocular pressure (IOP) that can be acute in nature with significant pain and corneal edema. Patients often present with a painful red eye, photophobia and decreased visual acuity.
Examination:
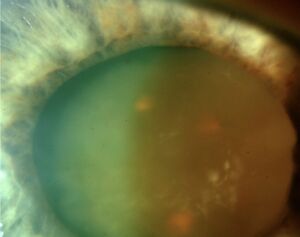
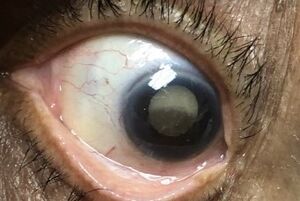
Slit lamp exam may reveal a pseudohypopyon composed of layered proteinaceous deposits. Close examination of the lens capsule may reveal wrinkling due to loss of lens mass with a mature/hypermature cataract (Figures 1 and 2). Gonioscopy, if corneal edema allows, should be performed to rule out presence of neovascularization and fibrovascular membranes (causing neovascular glaucoma) and dilated fundus examination should be completed, if visualization is possible, to identify posterior segment pathologies that can cause inflammation/neovascularization. Ultrasound testing should be used to examine the eye if visualization is not possible on dilated fundus exam. All of these steps are taken to rule out disease processes that might mimic (or potential coexist) with phacolytic glaucoma. The inflammation associated with leakage of proteins through the lens capsule may lead to posterior synechiae which will require attention at the time of cataract surgery, through lysing of the synechiae with various techniques, and can make extraction of the lens more difficult.
Differential Diagnosis:
There are other forms of open angle glaucoma that occur due to inflammation and the lack of keratic precipitates (KP) distinguishes phacolytic glaucoma from some of these other processes. Phacoantigenic glaucoma, a different form of lens induced glaucoma, is one example that exhibits inflammation with rise in IOP but does have coexistent KP. Uveitic glaucoma and neovascular glaucoma should also be part of the differential diagnosis as noted above.
Treatment:
The immediate goal is to decrease IOP and inflammation. Several topical IOP lowering drops are usually required to lower pressure including prostaglandin analogues, beta blockers and carbonic anhydrase inhibitors among others. Miotics, like pilocarpine, should be avoided as they might increase inflammation and formation of synechiae. Cycloplegics, like atropine, can be used to assist in breaking posterior synechiae and/or to avoid formation of new synechiae. Steroid drops can be used several times per day with the goal of bridging the patient to the definitive treatment which is cataract extraction. Cataract extraction should take place as soon as possible while still allowing for some time to control IOP and clear the edematous cornea when possible. It is important to note that combining glaucoma surgery, like trabeculectomy, with cataract surgery is not needed in this circumstance since cataract surgery alone is often “curative” unless the disease process has been present for a long period of time (months) and the outflow system of the eye has been severely compromised. A fortunate consequence of the pain and acute decrease of vision involved with phacolytic glaucoma is that patients often present to eye care professionals within days or weeks of symptoms thus allowing for intervention with standalone cataract surgery. There are exceptions of course, particularly in resource challenged areas around the globe, and consideration for combined cataract-glaucoma surgery should be considered when appropriate. Postoperatively, patients often must remain on IOP lowering drops for days to weeks along with steroids to decrease inflammation. Weaning off glaucoma drops is often possible and depends on the level of compromise to the aqueous outflow system. Every patient should undergo dilated fundus examination with optic nerve assessment as visualization improves to the back of the eye. Visual field testing can set a baseline for presence or absence of optic neuropathy and extended follow up, when possible, should be instituted to ensure recovery and addressing any future needs of the patient.
Lens Particle Glaucoma
WATCH LECTURE HERE: https://youtu.be/36LsOd7vJzs
Lens Particle Glaucoma results from liberated cataract material after the lens capsule is compromised post cataract surgery or post trauma. This is in distinction to phacolytic glaucoma which occurs in the absence of visible openings in the lens capsule. The liberated lens material subsequently acts as a physical barrier to aqueous humor egress from the anterior chamber across the trabecular meshwork and into the distal outflow system. Disruption of aqueous outflow leads to elevation of intraocular pressure (IOP) in a manner that is congruous with the amount of lens material present. The rise in IOP may present weeks to years after the presence of lens material in the anterior chamber.
Examination:
The typical presentation involves complaint of a red eye and photophobia with or without altered visual acuity. On exam, microcystic corneal edema is often present with lens material observable in the angle. The lens material is often composed of fluffy cortical material (Figure 3) with or without lens nucleus fragments (Figure 4). Moderate inflammatory reaction with cell and flare and synechiae formation are both common.
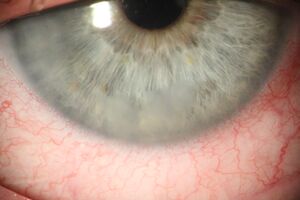
Differential Diagnosis:
Careful history and slit lamp examination will help direct the diagnosis and this is especially the case when past cataract surgery has been performed or ocular trauma has been reported with identifiable retained lens fragments. Phacolytic glaucoma, a form of secondary open angle glaucoma that is related to
leakage of high molecular weight proteins through microscopic openings in the capsule of a hypermature cataract, also results in elevated IOP but can easily be distinguished with history (lack of cataract surgery or trauma) and physical exam (presence of mature or hypermature cataract). Sending aqueous taps for analysis to identify polymorphonuclear cells could be done if there is confusion between lens particle glaucoma and phacoantigenic glaucoma, although the differences are academic since the treatment is essentially the same. Neovascular glaucoma and uveitic glaucoma may in part mimic the disease process of lens particle glaucoma but each can be ruled out with proper history and examination.
Treatment:
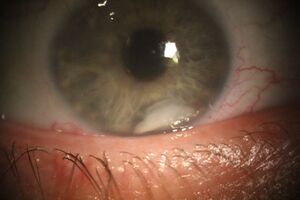
Fortunately, many of the patients who suffer from lens particle glaucoma do not actually manifest optic neuropathy if the disease is identified in early stages and prompt definitive treatment is instituted. Treatment is carried out with IOP lowering medications, mydriatics (to avoid or lessen formation of synechiae) and steroids until the lens material is resorbed or surgical intervention is completed to wash out the lens material. The surgical treatment involves anterior chamber washout with removal of all lens material from the anterior chamber. Every patient should undergo dilated fundus examination with optic nerve assessment at baseline. Visual field testing can set a baseline for presence or absence of optic neuropathy and extended follow up, when possible, should be instituted to ensure recovery and addressing any future needs of the patient.
Phacoantigenic Glaucoma
WATCH LECTURE HERE: https://youtu.be/7otwWM9-AXg
Phacoantigenic Glaucoma is a granulomatous inflammation resulting from sensitization to lens proteins post cataract surgery or trauma. The previously used descriptor was “phacoanaphylaxis”, however this term is no longer used since the disease process is not an allergic response. Occurrence is now uncommon (less than 1% of cataract surgeries) after introduction of modern phacoemulsification techniques which enhanced removal of most or all lens material at the time of cataract surgery. The immune response usually occurs within two weeks of surgery/trauma and involves an immune complex reaction mediated by IgG and the complement system.
The following 2 criteria must be satisfied before definitively accepting a diagnosis of phacoantigenic glaucoma:
1. Polymorphonuclear (PMN) leukocytes (Figure 5) must be present in the aqueous or vitreous specimen.
2. The circulating lens protein or particle content of the aqueous humor must be insufficient by itself to explain the glaucoma. In other words, the volume of lens material and debris are not sufficient to cause obstruction of the aqueous outflow system.
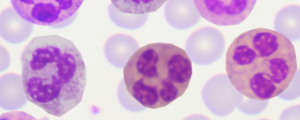
Sending aqueous taps for analysis to identify PMNs is not common and the diagnosis is often not definitively made prior to interventions being instituted.
Examination:
Mild to severe conjunctival hyperemia is usually present coupled with a decrease in visual acuity compared to early post-operative values. Slit lamp exam reveals keratic precipitates (KP) with low grade inflammation +/- vitritis and often with anterior/posterior synechiae. Gonioscopy may reveal lens material in angle, however the lens material may reside posterior to the iris and in the sulcus making ultrasound biomicroscopy helpful in the workup process. IOP may be initially lower due to inflammation inducing decreased aqueous humor production, but this is often followed with elevation in IOP and accompanying corneal edema.
Fortunately, glaucomatous optic neuropathy is not a common feature with proper identification and addressing the retained lens material often leads to resolution of the pathology. There is some evidence that this disease process is more common when lens material is intermixed with vitreous allowing for slow release of lens proteins over time.
Differential Diagnosis:
Careful history and slit lamp examination will help direct the diagnosis and this is especially the case when recent cataract surgery has been performed and retained lens fragments are identified. Lens-Particle glaucoma, which involves direct obstruction of the outflow material with retained lens fragments, is marked by a larger volume of lens material and the lack of PMNs in the aqueous humor and will be covered in a subsequent lecture. Neovascular glaucoma, uveitic glaucoma and phacolytic glaucoma may in part mimic the disease process of phacoantigenic glaucoma but as stated, each can be ruled out with proper history and examination.
Treatment:
Phacoantigenic glaucoma is treated with topical therapy to lower IOP as well as steroids to control inflammation. While it is possible for the disease process to resolve with topical therapy alone, removal of the lens protein, when significant, will both speed up recovery as well as result in definitive treatment. Miotics, like pilocarpine, should be avoided as they might increase inflammation and formation of synechiae. Cycloplegics, like atropine, can be used to assist in enhancing ocular comfort, breaking posterior synechiae and/or to avoid formation of new synechiae while other treatments are initiated to control pressure and inflammation.
The surgical treatment involves anterior chamber washout with removal of any bulk lens material once it is localized in the anterior chamber. A pars plana approach is needed for material in the anterior or posterior vitreous and a partial vitrectomy is often done to remove any microscopic lens material that surrounds larger particles. Every patient should undergo dilated fundus examination with optic nerve assessment at baseline. Visual field testing can set a baseline for presence or absence of optic neuropathy and extended follow up, when possible, should be instituted to ensure recovery and addressing any future needs of the patient.
Further Reading:
1. Ellant JP, Obstbaum SA. Lens-induced glaucoma. Doc Ophthalmol. 1992;81(3):317-38.
2. Epstein DL. Diagnosis and management of lens-induced glaucoma. Ophthalmology. 1982 Mar;89(3):227-30.
3. Epstein DL, Jedziniak J, Grant WM. Identification of heavy molecular weight soluble protein in aqueous humor in human phacolytic glaucoma. Invest Ophthalmol Vis Sci. 1978;17:398–402.
4. Richter C, Epstein DL. Lens-induced open-angle glaucoma. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas. 2nd ed. St Louis: Mosby; 1996.
5. Yanoff M, Scheie HG. Cytology of human lens aspirate. Its relationship to phacolytic glaucoma and phacoanaphylactic endophthalmitis. Arch Ophthalmic. 1968;80:166–170.
6. Kee C, Lee S. Lens particle glaucoma occurring 15 years after cataract surgery. Korean J Ophthalmol. 2001 Dec;15(2):137-9.
7. Jain SS, Rao P, Nayak P, Kothari K. Posterior capsular dehiscence following blunt injury causing delayed onset lens particle glaucoma. Indian J Ophthalmol. 2004 Dec;52(4):325-7.
8. Kim TH, Kim SJ, Kim E, Chung IY, Park JM, Yoo JM, Song JK, Seo SW. Spontaneous anterior lens capsular dehiscence causing lens particle glaucoma. Yonsei Med J. 2009 Jun 30;50(3):452-4.