Location of Glaucoma Drainage Implants and Tubes
| Primary authors |
|
|---|
Glaucoma drainage implants (GDIs) are an important treatment modality for glaucoma, especially for refractory types, such as neovascular and uveitic glaucomas. Organic materials (eg, horse hair and silk) or inert materials (eg, gold and platinum) were used as conduits to shunt aqueous from the anterior chamber to the subconjunctival space at the limbus as early GDIs prior to the 1900s.[1] The nonvalved Molteno implant was the first widely used GDI device of the modern era.[2] Shortly thereafter, Krupin developed a valved glaucoma implant to reduce the risk of early hypotony.[1] The Ahmed and Baerveldt GDIs were introduced in the 1990s and are now also widely used.[1]
Reasons for Glaucoma Drainage Implants
Although trabeculectomy with antifibrotic agents remains a common IOP-lowering glaucoma surgery worldwide, glaucoma patients with high risk for surgical failure (eg, uveitic and neovascular glaucoma) can achieve long-term intraocular pressure (IOP) control with GDI surgery. The Tube Versus Trabeculectomy (TVT) study’s 1-year results indicated comparable IOPs with GDIs compared with trabeculectomies, with a trend toward fewer reoperations in the GDI group.[3] The TVT study also suggested that GDIs are less likely to cause hypotony-related issues and are more likely to achieve IOP control compared with trabeculectomies.[3] GDIs also offer good IOP control in refractory glaucomas, such as those associated with uveitis, trauma, epithelial and fibrous down-growth, aniridia, and iridocorneal endothelial syndromes, whereas trabeculectomy is frequently less successful.[4]
Types of Glaucoma Drainage Implants
GDIs in popular use include the nonvalved single- and double-plate Molteno, the nonvalved Baerveldt, the valved Ahmed, and the valved Krupin glaucoma drainage implants.[1] The first widely used GDI was the Molteno implant, which has a round polypropylene end plate with a surface area of 134 mm2 for the single plate and 268 mm2 for the double plate GDI. The rounded plates of the double-plate Molteno implant are connected by a 10-mm silicone tube.[4] The Ahmed valved implant is made from either polypropylene (models S2, S3, and B1) or silicone (models FP7, FP8, and FX1).[4] Different plate sizes are available with surface areas ranging from 96 mm2 (S3 and FP8) to 184 mm2 (S2 and FP7) to 364 mm2 (B1 and FX1).[4] The Ahmed valve mechanism consists of 2 thin membrane-like elastomer sheets, which, in principle, restrict flow until a pressure of greater than 8 to 12 mm Hg is experienced intraocularly.[4] The Krupin slit valve consists of an oval silastic disc (13 × 18 mm)[5] with an area of 183 mm2[4] The pressure is regulated by horizontal and vertical slit valves. As an alternative, the tube end may be connected to a #220 silastic band. The Baerveldt nonvalved GDI plate is a barium-impregnated X-ray–visible smooth and flexible silicone plate that comes in 2 sizes—plate areas of 250 mm2 or 350 mm2 (the larger 500 mm2 model was discontinued).[6] The plate has fenestrations to allow bridging superior and inferior plate surface fibrous strands to develop, thereby reducing the vertical profile of the resultant bleb from 10.6 mm to 4.9 mm.[7]
Several GDIs offer multiple plate options, such as for the Molteno and the Ahmed. Various studies have shown that the larger the end plate size, the larger the surface area of encapsulation around the plate and thus the greater the degree of IOP reduction with a greater surface area of filtration.[8] Although larger surface area may be better for IOP lowering, an upper limit beyond which a larger size plate may not lead to lower IOP exists, and larger plates may lead to more surgical complications.[7]
The profile of each type of GDI ranges from a flatter 0.84 mm for the 2 Baerveldt plate sizes and 0.9 mm for the silicone FP7 Ahmed, to a higher profile for the Ahmed S2 (which is 1.9 mm high) and the Krupin (which is 1.75 mm high).[5][6] Capsule profile height is an important consideration because patient comfort and ocular motility may be decreased by greater plate height. Depending on the quadrant of GDI implantation, cosmesis may also be affected.
The composition of the plate affects capsule formation. A prospective, comparative study in which polypropylene Ahmed and silicone GDIs were compared showed a greater incidence of Tenon cysts formation in the poly-propylene group, and better IOP lowering was observed with the silicone plate group.[9] The more rigid polypropylene Ahmed plate is also believed to cause more movement of the plate against the globe, which can stimulate a more aggressive inflammatory reaction and thicker capsule wall formation associated with higher IOP outcomes. The more flexible silicone plate is suggested to reduce formation of thick filtering capsules. Also, valved GDIs have immediate fluid drainage to the plate, which may induce thicker capsule formation compared with nonvalved GDIs, with which aqueous flow is occluded until after capsule formation.[10] Cosmetically, this information is useful when choosing the quadrant of insertion for a GDI because thicker, higher capsules may be less desirable in the inferior quadrants. A lower-profile Baerveldt implant may be a better choice than an Ahmed for inferior quadrant placement.
Ocular Anatomy and Orbital Considerations for Glaucoma Drainage Implant Placement
Of the 4 orbital quadrants available for GDI placement, the most common and recommended site is the superotemporal quadrant. Key reasons for this recommendation include ease of plate implantation access in this quadrant for the surgeon, avoidance of the oblique extraocular muscles, and available conjunctival tissue for plate and tube coverage. The various GDI plate placement locations in the 4 orbital quadrants in relation to the limbus and the optic nerve were studied in human cadaver eyes. The Ahmed (S2 and FP7), Molteno (single-plate), and Baerveldt (250 and 350 mm2) GDIs were implanted onto cadaveric eyes with axial lengths ranging from 22.5 mm to 26.0 mm.[6] The measured variable was the maximum distance that a GDI could be placed posterior to the limbus in the various quadrants without encroaching within 2 mm of the optic nerve. The location of the GDI relative to the various muscles was also examined. The average maximum distance from the limbus to the anterior plate edge ranged between 9.0 and 15.0 mm in the superotemporal (ST) quadrant for all GDI types tested.[6]
For the other 3 quadrants, the distances spanned 8.0 to 14.0 mm, 9.0 to 14.0 mm, and 11.0 to 17.0 mm for the superonasal (SN), inferonasal (IN), and inferotemporal (IT) quadrants, respectively.[6] The “safe zone” from the plate to the optic nerve head was defined as 2 mm based on optic nerve changes found in rabbits after plates were implanted either abutting the optic nerve or within 1 mm of the nerve sheath.[11] Changes included microglial cell loss and localized astrocyte clustering.[11] Based on this study, the SN quadrant may offer the least amount of distance from the optic nerve for a GDI plate. This may be especially important for Ahmed GDIs, which have an anterior-posterior length of 16 mm compared with Molteno, Baerveldt 250 mm2, and Baerveldt 350 mm2 GDIs, having lengths of 13, 14, and 15 mm, respectively.[6] The width of the plate is also an important factor to consider. The Baervelt 250 mm2 GDI has a width of 22 mm, whereas the Baerveldt 350 mm2 GDI
has a width of 32 mm. The Ahmed, Molteno, and Krupin GDIs are 13 mm in width. The Baerveldt GDI plates, due to their wider plate widths, may be more likely to impinge on surrounding muscles, such as the rectus and oblique muscles. Such plate–muscle impingement may lead to patient dis-comfort and diplopia.
The IT quadrant is prone to more ocular muscle problems secondary to the close proximity of the inferior rectus and inferior oblique muscles. All GDIs, except for the Molteno, consistently contact the insertion site of the inferior oblique.[6] If a ST quadrant placement is not ideal, the next best quadrant for GDI placement is the inferonasal quadrant. Despite a potentially more difficult insertion due to exposure issues, an IN plate insertion is less likely to interfere with the adjacent inferior oblique muscle, as suggested by comparison of inferonasal to IT placement of Baerveldt 350 mm2 GDIs.[12] The variability of each patient’s eye means that surgeons should be aware of the differences in the “safe” range for GDI placement from eye to eye and quadrant to quadrant. This is especially important in nanophthalmos and in small eyes in hyperopes and children.[6]
Advantages and Disadvantages of Tube and Plate Locations
Previous ocular surgery is an important factor when considering GDI placement. Quadrants that have had previous ocular surgery, especially trabeculectomy with antifibrotic therapy, may have thin, friable conjunctiva, which may make conjunctival closure over the plate difficult. If a superior trabeculectomy is present, a GDI placed inferonasally may be a good option. In cases of a previous scleral buckling procedure, the GDI plate can be placed above the fibrotic band of tissue without the need for dissecting away fibrous tissue to access extraocular muscles. A fibrous capsule forms around the plate without difficulty, and some glaucoma surgeons routinely place Baerveldt GDI plates over the muscle. Although the capsule profile may be slightly higher, patients typically have good cosmetic results, IOP control, and minimal risk of diplopia.
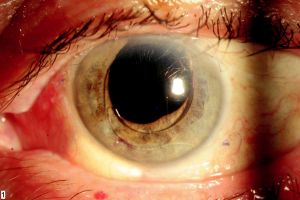
Our recommended site for placement of Molteno, Ahmed, or Baerveldt GDIs is the ST quadrant to avoid motility problems and for easier surgical access.[1] In cases of certain previous ocular surgery, such as trabeculectomy with antifibrotic agents or superior extracapsular cataract extraction (ECCE), the conjunctiva in the ST quadrant may be too scarred, thinned, or friable for placement of a superior GDI. In such cases, a GDI placed inferonasally may be appropriate (Figure 9-1). The IT quadrant is avoided for cosmetic reasons, as well as for the risk of diplopia or other motility problems associated with inferior oblique muscle dysfunction. Also, IN GDI placement may be preferable to a superior placement to avoid obstruction of the tube by silicone oil or egress of silicone oil into the subconjunctival space via the GDI, leading to significant inflammation.[1] The risk of hyphema (eg, in neovascular glaucoma) may preclude placing an inferior tube, as inferiorly layered blood may obstruct the filtering GDI tube tip, or the tube should be placed strategically longer for the tube tip to be higher within the anterior chamber.
The Ahmed S2 GDI has the highest profile at 1.9 mm and Baerveldt GDIs (both sizes) have the lowest profile at 0.84 mm.[6] The size of the plate for the various GDIs is important not only for IOP-lowering considerations but also in terms of where the implant can and should be placed. Molteno introduced the idea of draining aqueous to a posterior plate away from the limbus in 1969.[13][14] Although this led to decreased rates of exposure of the plate due to its posterior location, the risk of interference with extraocular muscles and even optic neuropathy from compression of the optic nerve increased. Similar to the Molteno GDI, the Ahmed GDI can be presumed to have similar plate characteristics in the various quadrants due to a similar plate profile and size (1.75 × 13 × 18 mm).[5]
The ST quadrant is the ideal plate location due to its combination of space, avoidance of the oblique muscles, and ease of exposure at the time of surgery. If the ST quadrant is not accessible, the IN quadrant is the next best site based upon our clinical experience. The IT quadrant is not an ideal location due to the potential for inferior oblique impingement leading to ocular motility disturbance. Also, large bleb formation inferotemporally can lead to an undesired cosmetic result. Although superior and inferior Ahmed GDI placements have similar results in terms of IOP reduction and preservation of vision, Pakravan et al[15] reported that inferiorly placed Ahmeds (which included a significant number placed inferotemporally) had more complications, including higher rates of wound dehiscence, tube or implant exposure, disfiguring encapsulation, lower lid bulge, exposure of the sclera patch graft, and endophthalmitis.
Anterior Chamber Tube Placement
Besides type of plate and plate placement location, the location of the GDI tube is also important. The GDI tube can be placed in different quadrants and different anterior-posterior chamber locations, such as anterior chamber (AC), sulcus, or pars plana (PP). AC tube placement is typically the most common location due to direct visualization and ease of placement during surgery. Postoperatively, the tube tip can be readily visualized to verify its placement and patency. Occlusion of the tube by iris, blood, fibrin, vitreous, and other ocular contents can be directly visualized for diagnosis and treatment of tube occlusion. An AC tube is also amenable for ripcord suture removal for early tube opening in the clinic. Also, if tube retraction is a concern, gonioscopy can be performed to verify the tube is in the AC. Some disadvantages to AC tube placement include corneal damage if the tube is in contact with the corneal endothelium, which can result in endothelial cell loss, corneal edema, and possible need for keratoplasty.[16] Eye rubbing can also cause areas of focal corneal edema due to intermittent tube–cornea contact. Another disadvantage is the difficulty of placing a tube in the AC in a quadrant where pre-existing high peripheral anterior synechia (PAS) is present.
The ST quadrant is ideal for AC placement of the tube, partly because it is the most spacious quadrant for plate placement and surgical site access during surgery. The tube is also easy to visualize with inferior gaze for laser suture lysis (LSL) through a corneal patch graft if early tube opening is necessary. For issues regarding patency of the tube or whether the GDI is filtering properly, the ST quadrant is relatively simple for ultrasound to visualize fluid over the plate and, at the slit lamp, a view of plate edge for the presence of a filtering bleb is relatively simple compared with other quadrants. A disadvantage of the ST location is that eye rubbing can more easily cause extrusion of the tube or erosion of the conjunctiva overlying the tube, especially in the early postoperative course. The risk of tube erosion can be minimized by routing the tube to enter the AC more superiorly toward the 12 o’clock meridian, which places the tube and patch graft entirely underneath the upper lid.[16]
If the ST quadrant is unavailable for GDI placement secondary to previous trabeculectomy, superior ECCE wound, or other reason for scarred or friable superior conjunctiva, the IN quadrant is our preferred next option for AC tube insertion. Adequate space exists inferonasally for most GDI plates and oblique muscles are avoided for a lower risk of diplopia. Tube erosion or extrusion is also less likely in the IN quadrant. If silicone oil is in the eye or may be needed in the future, the IN quadrant location of the tube minimizes the risk of silicone oil obstructing the tube. Some disadvantages of an IN tube include poorer cosmesis (the upper lid usually helps hide the tube in the ST AC), and hyphema can occlude an inferior tube tip if not above the level of the hyphema. For IN tubes, we typically use a transparent corneal patch graft to minimize cosmesis issues.
Placement of a tube in the AC in the SN and IT quadrants is often not ideal because of more difficult surgical site access for plate implantation, risk of diplopia, and cosmesis. Even implanting the plate in the ST or IN quadrants and routing the tube to the SN and IT quadrants is not ideal and may require use of a tube extender for optimal tube placement. The IT quadrant location is difficult due to possible interference with inferior oblique muscle function, hyphema obstructing the tube, and poorer cosmetic results with increased patch graft show.
Posterior Chamber Tube Placement
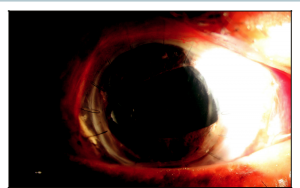
In eyes with a disorganized anterior segment, current or future need for keratoplasty, neovascularization of the angle, significant and/or high peripheral anterior synechiae, a shallow anterior chamber, history of PP vitrectomy, or future need for vitrectomy, a posterior chamber placement of a tube may be ideal.[17] A tube placed in the PP probably has a lower risk of erosion by virtue of the tube location being more posterior from the limbus and covered by Tenon’s and other soft tissue.[16] If a tube is placed in the PP, a complete vitrectomy to the vitreous skirt or base needs to be performed to prevent tube tip blockage by residual vitreous.[18] If blocked by vitreous, PP tube placement makes visualization of the tube tip difficult, and lasering the tube tip or needling the tube tip to remove the obstruction is typically not an option. A PP tube is also associated with a risk of retinal detachment, especially if significant inflammation occurs upon opening of a nonvalved tube with ligature release. Other contraindications of a PP tube include abnormalities of the sclera and lens status of the eye.[18] A higher postoperative hypotony risk was observed when a GDI tube was placed through a sclerostomy as opposed to AC placement.[18] When placing a tube in the PP (unless employing the Hoffman elbow for tube insertion), use of a 20-gauge microvitreoretinal blade creates a larger opening compared with a 23-gauge needle, and the resulting leakage around the sclerostomy site can lead to postoperative hypotony.
Sulcus placement of a tube may be an ideal compromise between an AC and a PP tube location in pseudophakic eyes. When a tube is placed in the sulcus, the tube is posterior to the iris, which keeps the tip below the iris plane and prevents tube contact with the cornea. This should prevent any direct corneal endothelial loss associated with tube corneal contact. A sulcus placement is also away from the vitreous and above the IOL, which typically prevents vitreous obstruction of the tube, especially with the tip bevel turned toward the IOL to minimize iris incarceration. Placing a tube in the sulcus may be more technically challenging. Ideal eyes for a sulcus tube placement are nonvitrectomy pseudophakic eyes with high PAS, such as neovascular or chronic angle-closure eyes (Figure 9-2).
Tube Entry Location
In certain cases, the length of the tube in a GDI may be inadequate after placement and may need to be relocated to a different position. This may occur in pediatric patients, in whom axial growth can lead to tube retraction from the globe.[19] Acute trauma, malposition of the tube, tube tip occlusion, accidently shortened tubes (eg, cutting in the operating room), and eye-rubbing–associated tube extrusion may require that the tube location be changed.[20] Various methods for relocating a tube exist, including using a connecting segment of 22-gauge angiocatheter tubing,[21]
a silastic sleeve,[22] a small-diameter silastic tube connected to the original tube,[23] or a commercially available tube extender, such as the Model TE (New World Medical, Inc).[20] A tube extender allows the surgeon to reroute a tube away from areas of high PAS or reoperated or scarred conjunctiva without having to explant an existing GDI and place a new GDI in a separate location for IOP control. Relocating an extruded tube is a relatively straightforward method to preserve a filtering GDI.
Key Points
- Our preference for GDI placement location is in the superotemporal quadrant, and in the inferonasal quadrant as the second choice for reasons including ease of surgical access, improved cosmesis, decreased risk of diplopia, access for laser suture lysis in nonvalved GDIs, and decreased risk of optic nerve damage.
- A larger glaucoma drainage plate size improves IOP control, but only to a certain point (ie, bigger is not always better and may increase the risks of complications such as diplopia).
- Anterior chamber GDI tube placement is preferred because visualization of the tube tip can aid in the diagnosis of poor or blocked filtration secondary to iris incarceration, fibrin, and other occlusions that are amenable to manipulation, such as laser treatment.
- Posterior chamber GDI tube placement is preferred in cases of a shal-low anterior chamber, other corneal problems (eg, Fuchs’ dystrophy, penetrating keratoplasty, or iridocorneal endothelial syndromes), or anterior segment problems that are planned for a vitrectomy. Sulcus GDI tube placement is often ideal for pseudophakic eyes with high PAS.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Dietlein TS, Jordan J, Lueke C, Krieglstein GK. Modern concepts in antiglaucomatous implant surgery. Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1653-1664.
- ↑ Minckler DS, Vedula SS, Li TJ, et al. Aqueous shunts for glaucoma. Cochrane Database Syst Rev. 2006(2):CD004918.
- ↑ 3.0 3.1 Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143(1):9-22.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17(2):181-189.
- ↑ 5.0 5.1 5.2 Krupin eye valve with disk for filtration surgery. The Krupin Eye Valve Filtering Surgery Study Group. Ophthalmology. 1994;101(4):651-658.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Kahook MY, Noecker RJ, Pantcheva MB, Schuman JS. Location of glaucoma drainage devices relative to the optic nerve. Br J Ophthalmol. 2006;90(8):1010-1013.
- ↑ 7.0 7.1 Lloyd MA, Baerveldt G, Fellenbaum PS, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101(8):1456-1463.
- ↑ Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99(10):1512-1519.
- ↑ Ishida K, Netland PA, Costa VP, et al. Comparison of polypropylene and silicone Ahmed Glaucoma valves. Ophthalmology. 2006;113(8):1320-1326.
- ↑ Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136(6):1001-1008.
- ↑ 11.0 11.1 Ayyala RS, Parma SE, Karcioglu ZA. Optic nerve changes following posterior insertion of glaucoma drainage device in rabbit model. J Glaucoma. 2004;13(2):145-148.
- ↑ Sidoti PA. Inferonasal placement of aqueous shunts. J Glaucoma. 2004;13(6):520-523.
- ↑ Molteno AC. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53(9):606-615.
- ↑ Molteno AC. New implant for drainage in glaucoma. Animal trial. Br J Ophthalmol. 1969;53(3):161-168.
- ↑ Pakravan M, Yazdani S, Shahabi C, Yaseri M. Superior versus inferior Ahmed glaucoma valve implantation. Ophthalmology. 2009;116(2):208-213.
- ↑ 16.0 16.1 16.2 Heuer DK, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma. 2001;10(6):493-496.
- ↑ de Guzman MH, Valencia A, Farinelli AC. Pars plana insertion of glaucoma drainage devices for refractory glaucoma. Clin Experiment Ophthalmol. 2006;34(2):102-107.
- ↑ 18.0 18.1 18.2 Scott IU, Alexandrakis G, Flynn HW Jr, et al. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol. 2000;129(3):334-341.
- ↑ Dawodu O, Levin AV. Spontaneous disconnection of glaucoma tube shunt extenders. J AAPOS. 2010;14(4):361-363.
- ↑ 20.0 20.1 Sarkisian SR, Netland PA. Tube extender for revision of glaucoma drainage implants. J Glaucoma. 2007;16(7):637-639.
- ↑ Smith MF, Doyle JW. Results of another modality for extending glaucoma drainage tubes. J Glaucoma. 1999;8(5):310-314.
- ↑ Kooner KS. Repair of Molteno implant during surgery. Am J Ophthalmol. 1994;117(5):673.
- ↑ Minckler D. Pathophysiology, indications, and surgical technique: glaucoma drainage devices. In: Weinreb RN, Mills RP, eds. Glaucoma Surgery: Principles and Techniques. San Francisco, CA: American Academy of Ophthalmology; 1998.
