Standard Technique for Implanting Glaucoma Drainage Devices
| Primary authors |
|
|---|
This chapter will review a basic step-by-step technique for implanta tion of non–flow-restrictive (eg, Baerveldt) and flow-restrictive (eg, Ahmed) glaucoma drainage devices (GDDs). The chapter will cover suggested instruments used to perform each step of the procedure, a description of how to perform each step of the surgery, the rationale for why each step of the procedure is performed, and a description of some pearls and pitfalls associated with each step of the procedure. The focus of this chapter is the description of the basic technique for GDD implantation. Please see subsequent chapters for a description of modifications of the techniques described below to address more complicated situations that one may encounter when performing GDD surgery.
Basic Steps to GDD Surgery Video Lecture: https://www.youtube.com/watch?v=lFrmQ5j9l6A
Obtaining Adequate Exposure
Adequate exposure is essential when performing GDD surgery. Two options are available for improving exposure. One option is to place a traction suture through the superior cornea. A 7-0 polyglactin suture is passed through the peripheral cornea at the 12 o’clock position. For inferior tube placement, an inferior corneal traction suture can be placed in a similar fashion at the 6 o’clock position. Care should be taken not to take too deep a pass and penetrate into the anterior chamber. If the patient has had a prior penetrating keratoplasty or other incisional corneal surgery, the corneal traction suture should be placed in a manner to avoid these sites. An alternative option to a corneal traction suture is to place the suture around the superior rectus muscle. After the traction suture is placed, the eye is rotated inferonasally to maximize exposure to the superotemporal bulbar conjunctiva. The traction suture is secured to the operative drape with a hemostat. A corneal protector is then placed over the cornea to protect it during the procedure and reduce retinal exposure to bright light from the operating microscope. Placement of viscoelastic beneath the corneal shield in patients with severe surface disease or corneal pathology can help to reduce the chance of a corneal epithelial defect developing during surgery.
Conjunctival Incision
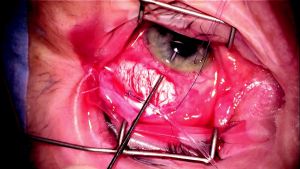
The technique I prefer is to create a conjunctival peritomy 5 mm posterior to the limbus. A caliper is used to measure 5 mm posterior to the limbus in the superotemporal quadrant. Using nontoothed forceps, such as Pierce-Hoskins forceps (Katena Eye Instruments, Denville, New Jersey) or Max Fine forceps (Altomed, Boldon, United Kingdom), the bulbar conjunctiva is gently tented up, and an incision is made through the conjunctiva with Westcott scissors. Using the Pierce-Hoskins forceps to hold up the conjunctiva adjacent to the incision, use Westcott scissors though the conjunctival incision to perform blunt dissection of the surrounding bulbar conjunctiva. Extend the initial conjunctival incision temporally and superiorly for 2 clock hours. The incision should follow the curve of the limbus. When extending the incision laterally and superiorly, the surgeon should be careful not to cut the superior or lateral recti muscles. Next, grasp Tenon’s capsule with the Pierce-Hoskins forceps and cut through it with Westcott scissors down to bare sclera. After Tenon’s tissue has been cut, it is best (whenever possible) to avoid grasping the conjunctiva until the time of closure so this tissue does not become damaged. Instead of grasping conjunctival tissue, grasp Tenon’s tissue with the Pierce-Hoskins forceps and use the Westcott scissors to perform blunt dissection to create a pocket in the superotemporal quadrant for the implant to be placed (Figure 8-1). Enter with the curved Stevens scissors (Katena Eye Instruments) and continue with blunt dissection. Adequate blunt dissection is achieved when one can insert closed curved Stevens scissors into the superotemporal quadrant and open them wide with little resistance. If bleeding is encountered during the blunt dissection, use cautery to achieve hemostasis. Instead of performing the conjunctival perimetry 5 mm posterior to the limbus as described above, an alternative technique is to create the conjunctival peritomy directly at the limbus.
Anesthesia
My preference for anesthesia is to provide a sub-Tenon’s injection of 3 to 4 cc of a 50/50 mixture of 2% lidocaine and 0.5% bupivicaine in the superotemporal quadrant around the superior and lateral rectus muscles. This should be done with a curved blunt-tip cannula (an olive tip works well). When injecting the anesthesia using the dominant hand, simultaneously use a Pierce-Hoskins forceps with the nondominant hand to press down on the conjunctiva, which helps trap the anesthetic in the desired location. An alternative means of anesthetizing the eye for the GDD surgery would be to perform a peribulbar or retrobulbar block prior to beginning the surgery.
Preparing the Glaucoma Drainage Device for Implantation
Ahmed Glaucoma Drainage Device
Ahmed glaucoma drainage devices (models S2, FP7, and S3) contain a built-in element that acts like a one -way valve to restrict the flow of aqueous through the implant. Before an Ahmed glaucoma drainage device can be implanted into the eye, it is necessary to first prime the tube. Priming involves injecting balanced salt solution through the tube to assure tube patency. A 27- or 30-gauge cannula is attached to the tip of the tube. Balanced salt solution is injected through the tube. As one presses down on the plunger to flush the tube with balanced salt solution, one should encounter moderate resistance. If there is little or no resistance (ie, balanced salt solution exits through the tube with little or no force exerted on the plunger of the cannula), the implant may be defective. If it is inserted into the eye and the flow restrictive element is not properly restricting flow, the patient is at risk for postoperative hypotony. If extensive resistance is encountered during flushing with balanced salt solution, this may also indicate a defective flow-restrictive element, and if the tube is implanted, it may be non-functional. In either case, if the tube does not flush properly, ask for a new GDD and send the defective one back to the manufacturer.
Baerveldt Glaucoma Drainage Device
Baerveldt glaucoma drainage devices (models BG 103-250 and BG 101-350) do not contain a flow-restrictive element. However, they still need to be primed with balanced salt solution to verify the patency of the tube. A 27-gauge cannula is attached to the tip of the tube. There should be no resistance when balanced salt solution is passed through the tube.
Next, a piece of 6-0 Prolene (Ethicon, Somerville, New Jersey) or 5-0 nylon suture is passed through the lumen of the Baerveldt implant.[1] This piece of suture will serve as a ripcord that will allow the surgeon a means of opening the implant at the slit lamp during the postoperative period, thus making it functional. Although some surgeons elect not to use a ripcord with this procedure, I routinely use ripcords because it allows me to control the precise timing of the opening of the tube. Without a ripcord, if the 7-0 polyglactin ligature obstructing the GDD is not easily accessable to be able to apply laser to dissolve the suture in the clinic during the postoperative period (because it is covered by a scleral patch graft), the tube will open spontaneously and the surgeon will be unaware if the intraocular pressure becomes too low, putting the patient at increased risk for complications associated with hypotony.
The final step in preparing a Baerveldt GDD is to tightly tie off the tube using a piece of 7-0 absorbable polyglactin suture so that no aqueous can exit through the tube until a capsule has formed around the plate. Because Baerveldt implants have no mechanism to restrict the flow of aqueous, if a capsule of tissue has yet to form around the plate, the patient will experience hypotony during the immediate postoperative period if the tube is not tied off completely. To help assure that the tube is completely tied off and will remain so until a capsule has formed, the tube is tied off with the 7-0 polyglactin suture by using at least 3 knots. I routinely use 6 knots to tie off the tube to be absolutely sure it will not open prematurely. Next, the cannula containing balanced salt solution is reattached to the tip of the tube. An attempt is made to forcefully pass balanced salt solution through the occluded tube. With forceful irrigation, if the surgeon is able to pass balanced salt solution through the occluded tube, this indicates that it is not completely occluded. Additional 7-0 polyglactin sutures should be placed adjacent to the first suture and tied off in the same manner described above.
Repeated testing with balanced salt solution should be performed until the surgeon is unable to forcefully pass any balanced salt solution through the tube, indicating it is completely occluded (Figure 8-2).
Securing the Plate of the Implant to the Sclera
Ahmed Glaucoma Drainage Device
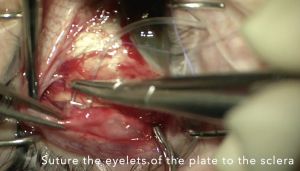
Ahmed GDDs are small enough that the plate can fit in between the superior and lateral rectus muscles. When grasping the Ahmed GDD to position it in the superotemporal quadrant, care should be taken to avoid touching the valvular element because it can easily get damaged. With the nondominant hand, the surgeon should use Pierce-Hoskins forceps to grasp and retract back the Tenon’s tissue, exposing the underlying bare sclera in the superotemporal quadrant. Using Nugent forceps, the surgeon should grasp the plate of the Ahmed implant behind the valvular element and place it in the superotemporal quadrant between Tenon’s tissue and bare sclera. When positioning the plate in the superotemporal quadrant, if resistance is encountered, one may need to perform additional blunt dissection using the Westcott or curved Stevens scissors. Next, the surgeon should use a caliper
to mark the sclera 8 mm posterior to the limbus in the superotemporal quadrant. It is important that the plate is secured at least 8 mm posterior to the limbus, as a more anterior placement of the plate can increase the risk of developing dellen during the postoperative period. Next, the surgeon should pass a 9-0 nylon suture through the sclera, at least 8 mm posterior to the limbus. Although it is important to take an adequate bite of sclera with the suture pass so that the suture does not cheese-wire through the sclera when it is tied off, one needs to be careful not to take too deep of a pass through the sclera so that the suture penetrates the choroid or retina. After taking the scleral bite, the suture should then be passed through the eyelet of the implant and tied off. A second 9-0 nylon suture should be passed through the sclera and then through the other eyelet and tied off. Tying forceps are then used to bury the knots within the eyelets to reduce the risk of them eroding through the conjunctiva during the postoperative period. After the plate is secured to the underlying sclera, calipers should be used to verify that the plate is situated at least 8 mm posterior to the limbus. If the implant is more anterior than 8 mm, one or both of the nylon sutures may need to be cut and resecured further back from the limbus.
Baerveldt Glaucoma Drainage Device
Baerveldt glaucoma drainage devices have a larger surface area relative to Ahmed glaucoma drainage implants and must be positioned under the insertions of the superior and lateral rectus muscles. The Baer-veldt 250 implant can be positioned with 1 wing under either the lateral or superior rectus muscle, whereas the Baerveldt 350 model is larger and needs to be positioned with both wings under the recti muscles. In general, this implant is designed to be placed underneath the extraocular muscles, but it can also be placed on top of the muscles in special circumstances, such as with prior strabismus surgery or traumatic globe injuries. In these cases, the normal extraocular muscle anatomy can be significantly disrupted, making standard placement underneath the muscles difficult or impossible.
Before attempting to place the implant in the superotemporal quadrant, the surgeon should use a muscle hook to identify and isolate the superior and lateral rectus muscles. It is important to clean and check ligaments beneath the muscles to ensure that the wing of the implant passes freely underneath the muscle. Next, with the nondominant hand, the surgeon should use Pierce-Hoskins forceps to grasp and retract back the Tenon’s tissue, exposing the underlying bare sclera in the superotemporal quadrant. With the dominant hand, the surgeon should next isolate and hook either the superior or lateral rectus muscles. After the muscle is hooked, the surgeon should hold the hooked muscle with the nondominant hand, freeing the dominant hand to be able to position the plate of the implant under the muscle. Using Nugent forceps, the surgeon should grasp the plate of the Baerveldt implant and place 1 wing of the plate behind the hooked rectus muscle. After the wing is situated behind the rectus muscle, the surgeon can release the hooked muscle and slide the muscle hook out from between the rectus muscle and wing of the plate. When removing the muscle hook, care should be taken to ensure that the wing of the Baerveldt remains properly positioned posterior to the rectus muscle.
When implanting the Baerveldt 350 model implant, the surgeon must next isolate and hook the second rectus muscle with the muscle hook. After this muscle is hooked, the plate of the implant is manipulated so that the second wing is positioned posterior to the hooked rectus muscle. When the wing is visualized behind the insertion of the rectus muscle, the hooked rectus muscle can be released and the muscle hook can be slid out from between the rectus muscle and the wing of the plate. Before securing the plate to the underlying sclera, the surgeon can check to verify that both wings of the implant are posterior to the recti muscles by grasping one of the eyelets with 0.12 forceps and tugging the plate toward the limbus. If both wings of the plate are posterior to the muscle insertions, resistance will be met when attempting to tug the plate more anterior toward the limbus. If there is little or no resistance with this maneuver, it may indicate that one or both of the wings may not be posterior to the rectus muscles, and the plate will need to be repositioned before it can be secured to the sclera.
Next, the surgeon should use calipers to mark the sclera 8 mm posterior to the limbus in the superotemporal quadrant. It is important that the plate is secured at least 8 mm posterior to the limbus, as a more anterior placement of the plate can increase the risk of developing dellen or plate erosions during the postoperative period. The surgeon should then pass a 9-0 nylon suture through the sclera, at least 8 mm posterior to the limbus. Although it is important to take an adequate bite of sclera with the suture pass so that the suture does not cheese-wire through the sclera when it is tied off, one needs to be careful not to take too deep of a pass through the sclera so that the suture penetrates the choroid or retina. After taking the sclera bite, the suture should then be passed through the eyelet of the implant and tied off. A second 9- 0 nylon suture should be passed through the sclera and then through the other eyelet and tied off. Tying forceps are then used to bury the knots within the eyelets to reduce the risk of them eroding through the conjunctiva during the postoperative period. After the plate is secured to the underlying sclera, the calipers should be used to verify that the plate is situated at least 8 mm posterior to the limbus. If the implant is more anterior than 8 mm, one or both of the nylon sutures may need to be cut and resecured further back from the limbus. For placement of Baerveldt GDDs in the inferonasal and inferotemporal quadrants, a similar technique is followed except that the wings of the implant are placed beneath the inferior rectus and corresponding medial or lateral rectus muscles.
Additional Conjunctival Dissection
The bulbar conjunctiva adjacent to the limbus can be thin and fri-able, and after it is dissected away from the underlying sclera it can lose its viability if it dries out. For this reason, I find it useful to hold off performing blunt dissection of this tissue away from the underlying sclera until after the plate is secured to the sclera. Using Westcott scissors, blunt dissection of the conjunctiva and Tenon’s tissue is performed all the way anterior up to the limbus. Additional blunt dissection can be performed by using Weck-Cel spears (Medtronic, Jacksonville, Florida) to gently lyse any additional adhesions that are present at the limbus. It is important to exert care when manipulating this tissue so it does not tear. If bleeding is encountered, cautery should be applied, when necessary, to achieve hemostasis. If there is scar tissue at the superotemporal limbus from prior intraocular surgery, the tube may need to be rerouted to adjacent tissue, which may be more viable. Likewise, if there is a prior trabeculectomy flap present in the superotemporal quadrant, care should be taken to avoid routing the tube through the flap of the trabeculectomy. Note, this step is not necessary if the initial conjunctival incision was performed at the limbus rather than 5 mm posterior to the limbus.
Trimming the Tube
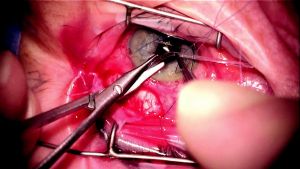
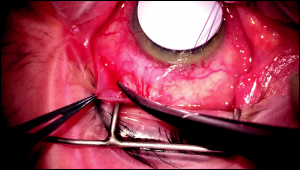
Corneal traction is released so that the eye can return to its normal position and the surgeon can determine the optimal location to trim the tube. Release of corneal traction is important because if the surgeon trims the tube while the eye is torqued inferonasally without accounting for this, the tip of the tube may end up too long in the anterior chamber after traction is released. If the tube needs to be rerouted around scar tissue, this should be taken into consideration when deciding the location to trim the tube, as it may need to be longer to account for such rerouting. With the nondominant hand, the tube is grasped with tying forceps and Westcott or Vannas scissors are used with the dominant hand to trim the tube in a manner so that a sharp bevel is created (Figure 8-3). It is important not to forcefully tug the tube toward the center of the cornea when grasping it with the nondominant hand prior to trimming it, as this can cause the surgeon to inadvertently trim the tube so that it is too short. When trimming the tube, one should err on the side of too long rather than too short. If it is too long, it can always be trimmed shorter, whereas if the tube is trimmed too short, a tube extender may be required to lengthen it.
If the plan is to insert the tube into the anterior chamber, one should trim the tube so that it will extend 2 to 3 mm into the anterior chamber with its bevel directed upward. If the tube is trimmed too short, there is a possibility it may retract posteriorly during the postoperative period and out of the anterior chamber. If the tube is trimmed too long, it increases the risk of the tip contacting the corneal endothelium, which would lead to corneal decompensation. If the plan is to insert the tube into the posterior chamber, the surgeon should create a downward rather than an upward bevel and should trim the tube so that it is longer. If the tube is cut short, it will be completely hidden behind the iris after it is inserted into the posterior chamber. By trimming the tube longer, it will be easier to visualize the tip of the tube during the postoperative period. If the plan is to insert the tube into the vitreous cavity to facilitate visualization of the tube tip, the surgeon should trim it so that it will extend at least 6 mm within the vitreous cavity. In eyes with prior vitrectomy, a longer tube in the vitreous cavity will also reduce the risk of tube occlusion from retained vitreous. This can happen in the setting of an incomplete vitrectomy where the vitreous skirt may not have been adequately trimmed prior to the tube insertion.
Paracentesis
A 75/15 blade or microvitreoretinal blade is used to create a paracentesis through temporal clear cornea. The purpose of creating a paracentesis is so that the surgeon can easily access the anterior chamber to reinflate the anterior chamber with balanced salt solution if it gets too shallow or to insert a cyclodiaylsis spatula in the event that the tip of the tube happens to get stuck in the iris during insertion. The paracentesis also gives the surgeon access to the anterior chamber in the early postoperative period to acutely lower or raise the intraocular pressure (IOP) by “burping” aqueous from the paracentesis or injecting viscoelastic through the paracentesis in the setting of profound hypotony.
Sclerostomy
Arguably, the most important step of the surgery is the creation of the sclerostomy, as this will determine where the tube will be positioned within the eye. The sclerostomy is created with a 22- or 23-gauge needle. An advantage of using a 22-gauge needle to create the sclerostomy is that it creates a larger sclerostomy so that it is easier to insert the tube into the anterior chamber. A disadvantage of a larger-size sclerostomy is that aqueous can exit through the sclerostomy around the lumen of the tube, increasing the risk of postoperative hypotony. For this reason, I prefer using a 23-gauge needle to create the sclerostomy. Although it creates a sclerostomy with a tighter fit, there is less room for aqueous to exit through the sclerostomy around the tube and thus a reduced risk of postoperative hypotony.
For implantation of the tube into the anterior chamber, I choose an entry site as anterior to the limbus as possible approximately at the 10:30 or 11 o’clock position whenoperating on a right eye; I use 1 o’clock for left eyes. If a buttonhole is present in the overlying conjunctiva at the planned site of the sclerostomy, I will create the sclerostomy at a different location to avoid the conjunctival buttonhole. If the plan is to place the tube into the posterior chamber, I create the sclerostomy 2 mm posterior to the limbus, whereas if the plan is to implant the tube into the vitreous cavity, the sclerostomy should be created 3.5 mm posterior to the limbus. When creating the sclerostomy, the 23-gauge needle should be grasped with its bevel upward. To assure proper positioning of the tube in the anterior chamber, it is helpful to enter with the needle into the anterior chamber following a pathway that is parallel to the plane of the iris (Figure 8-4). If the needle does not enter parallel to the iris plane, the tip of the tube may end up positioned against the corneal endothelium, which can increase the risk of corneal decompensation, or with the tube tip embedded into the iris stroma so that aqueous is unable to exit through the tube. If the patient is phakic, care should be taken when creating the sclerostomy not to enter too deep into the anterior chamber with the needle so as to risk damaging the lens or lens capsule. When placing the tube into the vitreous cavity, the needle should be oriented so the tube will be positioned just anterior to the center of the vitreous cavity.
Tube Insertion Through the Sclerostomy
Once the sclerostomy is created, the next step is to insert the tube through the sclerostomy. Gently retract the bulbar conjunctiva adjacent to the sclerostomy toward the limbus so it is not in the way during the tube insertion. Grasp the tube at its bevel with a tube introducer or angled Kelman forceps (Katena Eye Instruments) and insert the bevel completely into the sclerostomy. To facilitate the insertion, hold the bevel of the tube in the sclerostomy for 20 to 30 seconds before regrasping the tube slightly posteriorly and continue pushing the tube into the sclerostomy. Continue passing the tube through the sclerostomy until it can be visualized in the anterior chamber. Watch the tip of the tube as it enters into the anterior chamber to be sure that it is properly positioned in the anterior chamber. If the tip of the tube gets embedded in the iris, it can often be repositioned by inserting a cyclodialysis spatula through the paracentesis and sweeping the tip away from the iris. If the tube tip is visualized abutting the peripheral cornea, the tube will need to be pulled back out through the sclerostomy, the sclerostomy sutured shut, and a new sclerostomy created. If the tube tip is visualized to be too long, it should be pulled back out of the sclerostomy, trimmed to the proper length, and reinserted.
Securing the Tube to Sclera
To aid in the remaining steps of the surgery, the eye should again be rotated inferonasally and held in this position using the traction suture.
Once the tube is visualized to be properly positioned within the eye, the exposed portion of the tube is secured to the underlying sclera using a 9- 0 nylon suture by creating a figure-of-eight knot. Care should be taken not to penetrate the lumen of the tube when passing this suture.
Venting Slits
Because Baerveldt implants are completely tied off, until the ripcord is pulled and the tube is opened or the tube opens spontaneously, the IOP will continue to remain elevated during the immediate postoperative period. Some surgeons elect to make 1 to 5 small punctures into the lumen of the tube between the sclerostomy and the plate that serve as venting slits to help lower the IOP during the immediate postoperative period.[2] The venting slits are created by using a spatulated needle. A TG-140 needle works well.
Covering the Tube With a Patch Graft
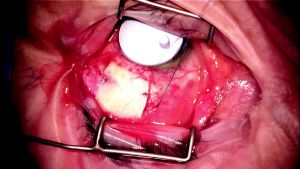
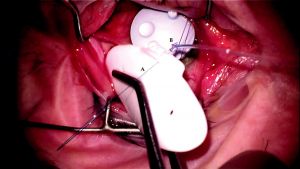
To prevent the tube from eroding through the overlying bulbar conjunctiva, it is strongly recommended to cover the exposed tube from the plate to the sclerostomy with a patch graft.[3] Different materials including donor sclera, donor cornea, or Tutoplast (New World Medical, Inc) can be used as a patch graft to cover the exposed tube. The most important portion of the tube requiring coverage with the patch graft is the site where the tube enters into the sclerostomy. The patch graft can be tacked down to the underlying sclera by using 2 to 4 7-0 polyglactin sutures (Figure 8- 5). When securing the patch graft to the underlying sclera, care should be taken not to penetrate the lumen of the tube.
Conjunctival Closure
Prior to reapproximation of the conjunctival wound during Baerveldt implant surgery, one will need to trim the ripcord and tuck it under the bulbar conjunctiva before closing the conjunctival wound if the ripcord technique was used. The ripcord should be tucked under the conjunctiva in a location where it is easy to get to at the slit lamp, such as under the temporal bulbar conjunctiva, close to the limbus.
To reapproximate the conjunctiva, the posterior edge of the bulbar conjunctival wound is grasped with 2 Pierce-Hoskins forceps and gently shimmied forward so it can be sutured to the anterior edge of the bulbar conjunctival wound. Two to 3 evenly spaced interrupted 8-0 polyglactin sutures on a vascular needle are used to secure the 2 edges of the conjunctival wound. Next, the conjunctival wound is reapproximated by using a running locked 8-0 polyglactin suture on a vascular needle. During wound closure, it is very important to be sure that one is reapproximating bulbar conjunctiva to bulbar conjunctiva and not conjunctiva to Tenon’s tissue.
Completion of the Surgery
At the conclusion of the surgery, one should inspect the depth of the anterior chamber and evaluate whether the IOP is physiologic. The traction suture is removed. A fluorescein strip is used to check the conjunctival wound and paracentesis site for leaks. A subconjunctival injection of steroids and antibiotics is given inferiorly. In cases of uveitic or neovascular glaucoma, a drop of atropine 1% can be helpful to help maximize the anterior chamber depth and stabilize the blood-ocular barrier. The eyelid speculum is removed. The eye is dressed with bacitracin or erythromycin ointment and closed. A patch and metal shield are placed over the eye for protection.
Key Points
- When preparing a non–flow-restrictive implant such as a Baerveldt GDD or Molteno GDD, is it essential that the tube is completely tied off before such devices are implanted into the eye. Without complete ligature of these devices, all of the aqueous can escape through the GDD, resulting in hypotony with a shallow or flat anterior chamber. These patients are at significantly increased risk for experiencing serious sight-threatening complications such as suprachoroidal hemorrhage.
- One of the most important steps of GDD surgery is the creation of the sclerostomy though which the GDD will course into the eye. The entry should be parallel to the plane of the iris. An entry that is too anterior can result in the tip of the tube adjacent to the cornea, increasing the risk of corneal decompensation. If the entry is too posterior, the tube tip may become embedded in the iris.
- When trimming the GDD to the proper length, one should err on the side of leaving the tube tip too long, as it is much easier to trim the tube further than to risk creating a tube that is too short.
- When covering the tube with a patch graft, it is important to attain complete coverage of the exposed tube from the sclerostomy site to the site where the tube attaches to the plate.
References
- ↑ Lerner SF, Parrish RK. Glaucoma aqueous humor drainage devices. In: Chen TC, ed. Glaucoma Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:111-125.
- ↑ Fechter HP, Lee PP, Walsh MM. Non-valved single-plate tube shunt procedures: Baerveldt and Molteno implants. In: Chen TC, ed. Glaucoma Surgery. Philadelphia, PA: Elsevier Inc.; 2008:87-121.
- ↑ Raviv T, Greenfield DS, Liebmann JM, Sidoti PA, Ishikawa H, Ritch R. Pericardial patch grafts in glaucoma implant surgery. J Glaucoma. 1998;7(1):27-32.