Trabeculectomy Surgery: Decision Making and Technique
| Primary authors |
|
|---|
The goal of glaucoma filtration surgery is to decrease the intraocular pressure (IOP) to a level that will arrest or retard the loss of the nerve fiber layer. The unifying feature of all glaucoma filtering surgery is to create a drainage system that flows into a newly created sub-Tenon’s/conjunctival reservoir, the “bleb.” The creation of a bleb allows the aqueous to bypass the nonfunctioning or, more commonly, poorly functioning trabecular mesh-work and thereby lower the IOP.
Preoperative Assessment
Typically, the surgical option becomes a viable management alternative in the presence of advancing visual field defects and/or progressive loss of retinal nerve fiber layer, despite maximal tolerated medical therapy and laser trabeculoplasty (when appropriate).
Risk factors for surgical failure and the quality of postoperative care should be considered prior to filtration surgery. The risk factors for filtration failure[1][2] include young patients (except for young myopes <50 years old, who are at risk for overfiltration and hypotony maculopathy), diabetes, higher preoperative IOP, African American patients, iris/angle neovascularization, uveitis, and prior failed filtration surgery. The patient and/or caretakers must understand that the postoperative care is just as important as the surgery itself and that all postoperative instructions must be followed. Poor postoperative compliance can lead to prolonged uveitis, posterior synechia, cystoid macular edema, and bleb failure.
Additional Perioperative Considerations
Hyperopic Eyes
- Prone to shallow/flat anterior chambers postoperatively. Consider tighter scleral flap closure to avoid postoperative hypotony and shallow/ flat anterior chamber.
- When associated with chronic angle closure, there is an increased risk of malignant glaucoma.
Pseudophakic (Postoperative) Eyes
- Check conjunctival mobility prior to choosing a filter site as prior cataract surgery might lead to conjunctival scarring if performed through a scleral tunnel.
- Use caution when creating a scleral flap through an old cataract wound incision because there is a risk of scleral flap avulsion.
- Perform anterior vitrectomy for vitreous in the anterior chamber.
Perioperative Systemic Medications
Oral steroids may be useful in patients with uveitic glaucoma but should only be used in selected cases and preferably after consultation with an internist or uveitis specialist. Intraocular inflammation at the time of surgery is highly associated with bleb failure.
If possible, both anticoagulants and antiplatelet agents should be stopped to decrease the risk of intraoperative, retrobulbar, or suprachorial hemorrhage. Cessation of blood thinners should be done only after consultation with the patient’s internist or cardiologist.[3] Many surgeons choose not to discontinue these medications due to the elevated risk of vascular events. This issue should be discussed thoroughly with the patient.
Basic Surgical Technique
Glaucoma filtration surgery is performed in either the superonasal quadrant or directly superior, leaving the superotemporal quadrant available for a repeat filtration surgery or the use of a glaucoma drainage device. Anesthesia is typically accomplished effectively with a retrobulbar block consisting of a combination of lidocaine, bupivacaine, and hyaluronidase. Lid blocks and general anesthesia are rarely required.
Traction Sutures
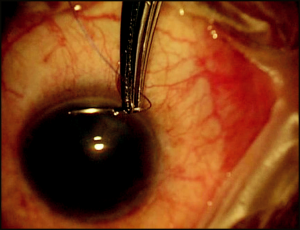
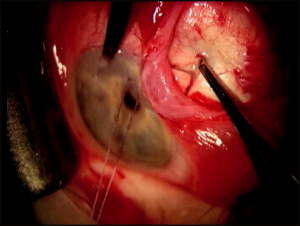
A superior corneal bridle suture is the common approach used to rotate the eye inferiorly (Figure 4-1). This is performed with a 6-0 or 7-0 Vicryl or silk suture on a spatulated needle passed through clear, midstromal cornea approximately 1 mm from the superior limbus for approximately 2 to 2.5 mm. Gentle traction on the suture assures its integrity prior to taping or clamping the suture to the inferior drape or handing it over to an assistant to hold. An alternative approach is to place an inferior corneal bridle suture.
Avoid passing the needle into the anterior chamber, which produces a persistent aqueous leak at the suture site that could subsequently lead to intraoperative hypotony, a shallow anterior chamber, and possibly prevent the use of mitomycin C (MMC) due to the risk of intraocular tracking.
Creation of the Conjunctival Flap
The conjunctival flap may be either limbus- or fornix-based. Evidence suggests that both are successful.[4] However, debate continues regarding which is more efficacious. With either technique, the conjunctival tissue will ultimately function as a fluid flow resistor. It is therefore important to avoid excessive tissue manipulation, which can cause subconjunctival fibrosis, the most common cause of filtration failure. Only nontoothed forceps should be used on this tissue.
Limbus-Based Flap
Limbus-based conjunctival flaps are less likely to leak postoperatively, reducing their likelihood of flattening or scarring down.
It is necessary to make the initial conjunctival incision parallel to the eyelid margin, a minimum of 8 mm posteriorly. The Tenon’s fascia, in turn, is grasped and incised until the episclera is visualized. The conjunctival– Tenon’s wound should be lengthened to approximately 2 clock hours. The conjunctiva and Tenon’s tissue should only be incised when in the grasp of forceps and while they are raised over the episclera to avoid incorporating a rectus muscle with these tissues.
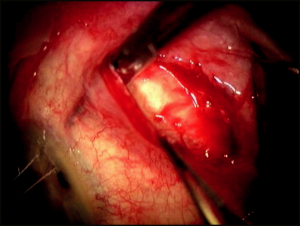
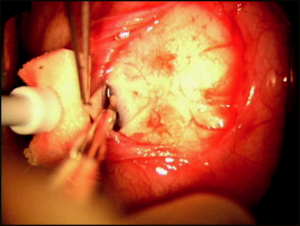
The conjunctival–Tenon’s flap is bluntly dissected to the limbus through the insertion of Tenon’s fascia (approximately 0.5 to 0.75 mm posterior to the limbus) to the insertion of the conjunctiva, which is approximately 0.5 mm onto clear cornea (Figure 4-2). Meticulous hemostasis using tapered tip cautery is then performed.
Fornix-Based Flap
Fornix-based conjunctival flaps have the advantage that they are easier to perform and can be used as an alternative to the limbal-based flap (Figure 4-3). These are initiated with a 1.5 to 2 clock-hour limbal peritomy and blunt dissection, which is carried posteriorly using blunt-tipped Westcott scissors through the insertion of Tenon’s fascia. Blunt dissection is then carried posteriorly and laterally as far as the Westcott scissors can reach to obtain the most diffuse bleb possible.
Creating a Scleral Flap
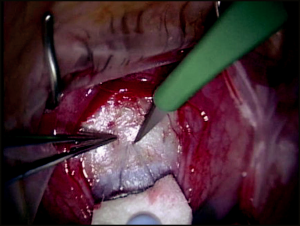
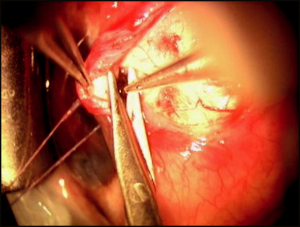
The scleral flap, if adequately sutured, is a temporary resistor to the flow of aqueous through the sclerotomy site in the early postoperative period, reducing the incidence of hypotony. The shape of the scleral flap is of little consequence as long as the flap completely covers the sclerectomy. Our preference is to make a triangular flap 3.5 mm × 3.5 mm × 3.5 mm with a 15-degree blade, with a flap thickness of one-half to two-thirds sclera thickness (to avoid flap avulsion) (Figure 4-4). The flap is dissected anteriorly, beyond the gray line and into clear cornea anterior to the sclera spur and ciliary body, into clear cornea in a lamellar fashion with the same blade.
Mitomycin C
MMC is an antifibrotic agent used intraoperatively to reduce postoperative subconjunctival scarring, thus reducing the need for or eliminating multiple postoperative subconjunctival injections of 5-fluorouracil (5-FU), which historically was the antifibrotic agent used routinely prior to the wide-spread usage of MMC. When compared to 5-FU, MMC produces less corneal toxicity.[5] However, a dose-dependent complication associated with MMC chamber paracentesis.
administration is long-term postoperative hypotony with associated maculopathy,[6] most commonly due to an avascular thin-walled bleb that over-filtrates. However, aqueous hypo-secretion secondary to ciliary body toxicity may play a role in some cases.[7]
The concentration of MMC (0.2 to 0.5 mg/mL) used and the duration (2 to 5 minutes) of application during glaucoma filtration surgery varies in the literature.[8][9][10] To reduce the risk of long-term hypotony with maculopathy, the duration of MMC exposure should be adjusted according to the risk factors of the individual patient.
Because MMC can be highly toxic to the corneal endothelium and tissues of the anterior segment,[11][12] it should be applied in a controlled manner to bare sclera prior to entering the eye. A cellulose or cut end of a Weck-Cel sponge saturated with MMC is applied to bare sclera. We recommend diffuse application of multiple MMC sponges, under all areas of Tenon’s fascia, to decrease the incidence of high avascular localized blebs and to promote low diffuse filtering blebs. After the exposure period, the sponge is removed and the surgical site is thoroughly irrigated with a full container (15 mL) of balanced salt solution.
Paracentesis
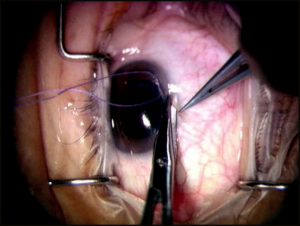
Placement of a temporal-beveled, self- sealing paracentesis during filtration surgery before the sclerotomy but after application/rinsing of an antifibrotic agent is essential in allowing the surgeon access to the anterior chamber, similar to cataract surgery (Figure 4-5). A paracentesis enables the surgeon to allow for gradual pressure decline in patients in whom the IOP is high (reducing the risk of suprachoroidal hemorrhage), and to reform the anterior chamber to assess for adequate filtration at the flap margins after sclera flat closure.
Sclerectomy
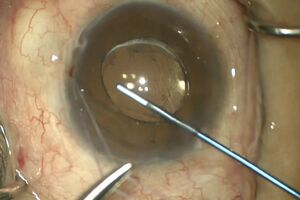
There are 2 common methods for making the sclerotomy opening. For both methods, the anterior chamber is entered at the anterior most point of the scleral bed adjacent to the scleral flap with a 15-degree blade, or other such sharp knife. For the first method, 2 radial incisions centered under the scleral flap are made approximately 1.5 to 2 mm apart with Vannas scissors. The block is retracted posteriorly and excised with Vannas scissors. Alternatively, a sclerectomy can be made with a Kelly-Descemet punch (our preferred method). Two to 3 punches may be required to make a sclerectomy of adequate size (Figure 4- 6). If the iris balloons forward through the surgical opening at any time during the construction of the sclerectomy, a small radial snip of the iris with Vannas scissors can deflate the ballooning.
Iridectomy
A peripheral iridectomy is performed to prevent obstruction/incarceration of the iris in the sclerectomy. The ideal iridectomy should be larger than the sclerectomy in all dimensions (wide at its base but short vertically to avoid iatrogenic polycoria and its associated monocular diplopia). With the scleral flap lifted, the iris is grasped 0.5 mm from the iris root and retracted through the sclerotomy. The scissors are opened enough to encompass the retracted iris, and then in one smooth cut, the iridectomy is made (Figure 4-7). The iris is reposited with a stream of balanced salt solution or by closing and gently massaging over the scleral flap. Upon completion of the iridectomy, the surgeon should have a view of the ciliary processes and occasionally the lens equator. If iris remnants or ciliary processes occlude the sclerectomy, these should be excised only with great caution because it is exceedingly easy to damage the lens or hyaloid face.
Scleral Flap Closure
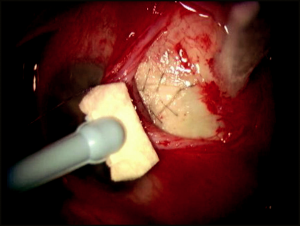
The scleral flap should be closed tightly enough to prevent postoperative hypotony. The flap is closed with interrupted 10-0 nylon sutures. A 3-1-1 knot buries well and secures the flap adequately. Usually 3 to 5 sutures are used to adequately close the flap (Figure 4-8). After the flap is secured, the anterior chamber is reformed through the paracentesis with balanced salt solution, and the filtration is checked at the flap mar-gins with a Weck-Cel sponge. If the IOP and anterior chamber depth are maintained with slow oozing of aqueous humor, then the scleral flap clo-sure is usually adequate. However, if aqueous humor flows freely and the anterior chamber shallows, additional sutures are required. Conversely, if aqueous humor does not flow, loosen, remove, or replace sutures. Also, it may be necessary to reopen the scleral flap and inspect the sclerectomy to ensure it is not obstructed.
Conjunctival Closure
Watertight conjunctival closure using nontoothed forceps is necessary to create an elevated filtering bleb. Tissues should be brought to apposi-tion only, as tight sutures “cheese-wire” postoperatively, creating a leaky, inflamed wound. Meticulous closure of the conjunctiva can save many postoperative hours dealing with the complications related to poorly closed wounds.
Limbus-Based Flap Closure
While the terminology is confusing, a limbus -based flap is performed with a conjunctival and Tenon’s incision, typically performed in the superi-or fornix, approximately 8 to 10 mm posterior to the limbus. Most surgeons favor the use of running conjunctival closures with 8-0 or 9 -0 absorbable suture (eg, Vicryl) on a BV needle, beginning on the side of the surgeon’s dominant hand. The running suture closes the Tenon’s fascia first, followed by the conjunctiva (Figure 4-9). Weck-Cel sponges are then used to assure the wound’s watertight closure. It is useful to lock the running suture every second to third throw to provide watertight closure. Care should be taken not to take large bites of the anterior Tenon’s fascia or conjunctiva, as this may cause the wound to migrate anteriorly, creating unwanted tension on the limbal conjunctiva.
Fornix-Based Flap Closure
While the terminology is confusing here as well, a fornix-based flap is created by incising the conjunctiva directly at the limbus, and dissecting posteriorly beneath Tenon’s. Fornix-based flaps should be closed in a water-tight manner as well. Closure with winged sutures using nylon or Vicryl at either end of the conjunctival flap positions the leading edge of the flap over the limbus. Alternatively, if this closure is inadequate, 3 to 4 long mat-tress sutures are placed at the limbus using 10- 0 nylon or 8-0 to 10-0 Vicryl on a spatulated needle. The suture should be placed through midstromal cornea. Exposed nonabsorbable sutures are removed after wound healing has occurred.
Seidel testing for bleb leaks with a saturated fluorescein strip should be performed at the conclusion of fornix-based surgery. If a bleb leak is detected, it should be closed with a single suture or a horizontal mattress suture and the wound rechecked with fluorescein.
Intraoperative Complications
Buttonholes
Buttonholes can generally be avoided by meticulous handling of the conjunctiva with nontoothed forceps. If a large buttonhole overlying the filter site is found early in the surgery, the surgeon should consider relocat-ing the filter to the adjacent quadrant. If the buttonhole is found or created late in the surgery, take care to not extend it while completing the surgery. Then make the repair with a tapered 9- 0 or 10 -0 Vicryl or nylon suture on a tapered needle in a mattress fashion, taking care to incorporate Tenon’s tissue in your needle passes for stability, if possible.
Flap Dehiscence
A thin scleral flap is at risk for avulsion from the eye during the surgery. If done before making the sclerotomy, it is recommended to abandon that site and begin again in an adjacent area. If done after the sclerotomy, it is recommended that a scleral patch graft be placed over the sclerotomy and closed in watertight fashion, abandoning the filtration.
Bleeding
Bleeding from the sclerotomy edges and the iridectomy edges is not uncommon. Gentle cautery of small bleeders can be performed under direct visualization with taper tip cautery. To stop persistent bleeding, epinephrine 1:100,000 solution (sterile and unpreserved) can also be used. Take care to not inadvertently enlarge the sclerotomy site, as this can cause overfiltration. In addition, cautery of the iris should be performed with caution so as to not break the anterior hyaloid face and encounter vitreous or to violate the lens capsule in a phakic patient.
Subconjunctival Injections
At the conclusion of filtering surgery, subconjunctival dexamethasone phosphate 5 mg is injected opposite the site of the filtering bleb. Injec anti-biotics can be used, but some surgeons feel it may increase postoperative inflammation. Cycloplegics (eg, atropine 1%, homatropine 5%, or scopol-amine 0.25%) may be given at the conclusion of the surgery if the patient is still phakic or has a shallow chamber to begin with. This is followed by a combination steroid/antibiotic ointment (eg, Tobradex or Maxitrol), which is then applied. The eye is then gently patched and an eye shield is applied.
Postoperative Management
Topical steroids improve intermediate and long-term postoperative IOP control after filtering surgery by decreasing postoperative inflammation and scarring. Prednisolone acetate 1% is administered every 1 to 2 hours initially and tapered over 8 to 12 weeks, depending on the patient’s response. No additional benefit has been demonstrated with the use of systemic pred-nisone.[13] Cycloplegics (eg, scopolamine 0.25%) can be used twice a day for 2 to 3 weeks after surgery to help maintain anterior chamber depth and prevent synechia. Antibiotics are given over a 2-week period. In addion, an antibiotic/steroid ointment is generally given at bedtime to aid in antibiotic and anti-inflammatory coverage while asleep.
It is important to understand that postoperatively there could be changes in the medical regimen that may affect the IOP in the fellow eye due to steroid response,[14] discontinuation of oral glaucoma medication, and confusion with medications. If the IOP reaches unsafe levels in the fellow eye, the surgeon must advance therapy accordingly.
Postoperative Scleral Flap Suture Release
If the IOP is not adequately lowered, laser suture lysis is a technique that allows the surgeon to modify scleral flap aqueous humor outflow resis-tance postoperatively.[15] With this available, the scleral flap can be closed more tightly intraoperatively, thus reducing the occurrence of postopera-tive hypotony and its attendant complications without as much concern for postoperative elevated IOP.
Suture lysis is performed sequentially; that is, 1 suture at a time. The time interval between cutting sutures can be from hours to days, depending on the response. Suture lysis can be performed as early as 1 week after filter-ing surgery and as far out as 18 weeks after surgery.[16]
After application of topical anesthesia, a Zeiss, Hoskins, or Blumenthal lens is used to flatten the bleb overlying the scleral flap. The lens may need to be held in place for a short period of time (usually under a minute) to com-press the bleb tissues and improve the view of the suture before laser applica-tion (Figure 4-10). An argon red or green wavelength is used with settings of 240 to 400 mW, 0.1 seconds, and a 50 μm spot size (see Appendix E for alternative laser settings). Argon red is useful if subconjunctival hemorrhage or pigmentation is present due to its reduced absorption by these substances. The suture should clearly separate when successfully cut. If there is no clear separation of the suture, it was not cut or it was not functionally closing the scleral flap. Additional sutures may need to be cut in the latter situation. Occasionally, a cut end stands vertically inside the bleb after lysis. This can be avoided if the suture is cut at its 2 ends. Releasable sutures, a method to remove scleral flap sutures at the slit lamp (beyond the scope of this chap-ter), can be used to circumvent the need for a laser.
After lysing or pulling a suture, it is important to judge the patency of the flap by using digital pressure (through the superior lid) or the Carlo Tra-verso maneuver (pressure using a cotton applicator adjacent to but outside the flap boundary)[17] to determine whether there is an elevation in the bleb after suture lysis.
The Failed Filter
Unfortunately, even surgery performed and followed in the most meticulous manner may fail. Failure occurs when aqueous humor meets elevated resistance between the sclerectomy and the conjunctival epithelium.
Signs of bleb failure include the following:
- Increased bleb vascularity.
- Increased bleb wall thickness.
- Decreased elevation of the bleb.
- Reduction in conjunctival microcysts.
- Increased IOP.
Causes of bleb failure include the following:
- Subconjunctival (episcleral) fibrosis (most common cause).
- Scleral flap fibrosis
- “Tenon’s cyst” formation (a fluid-filled cavity lined internally by fibrous tissue preventing filtration).
- Obstruction of the sclerectomy by iris, vitreous or lens (as seen by gonioscopy).
Therapeutic options include the following:
- Postoperative topical anti-inflammatory drop adherence, early digital massage, and 5-FU injections (for causes 1 and 2 prior to formation).
- External bleb needle revision (for causes 1, 2, and 3).
- Nd:YAG or argon laser reopening of the sclerectomy (for cause 4).
Digital Massage
Digital massage is used to push aqueous through the filter site in an attempt to prevent or slow the fibrosis that causes filtration surgery to ultimately fail.
While the patient is looking up and nasally, the index finger is used to identify the infraorbital rim. The patient should then place pressure on the globe through the lower lid with pressure directed inward and upward for 10 seconds with the soft fingerprint portion of the index finger. This is repeated 1 to 2 times per day until the patient is seen in clinic to reassess the filtering site. Risk is involved with this maneuver, and specific instructions and cautions must be given to ensure that the patient does not cause injury to him or herself. For this reason, many physicians choose not to use this manuever.
Bleb Needle Revision
In cases of bleb failure resulting from an elevated thick-walled encap-sulated bleb (Tenon’s cyst) or from advanced episcleral fibrosis causing a flat bleb, a needling procedure[18] may be used in an attempt to successfully revise the original filtering surgery. This is performed using a TB syringe to draw up 0.1 mL of sterile nonpreserved 1% lidocaine and 0.1 mL of 0.4 mg/mL MMC, yielding a final concentration of 0.2 mg/mL of MMC. Under sterile technique in the operating room or at the slit lamp, the diluted MMC is injected subconjunctivally in the superotemporal quadrant, far from the failed bleb (in the case of a superonasal bleb). If at the slit lamp, massage the MMC through the closed eyelid, toward the site of the initial surgery, until the area is flat. If in the operating room, you may use a cot-ton tip applicator on the conjunctiva to accomplish this flattening. A 25- or 27-gauge needle on a TB syringe or a 1.0 mm side-port blade is then inserted subconjuctivally, far from the bleb and away from the area of the MMC, and advanced until reaching the failed bleb. The sharp edges are then used to break up scar tissue, lift the flap, and enter the anterior chamber through the sclerotomy site to reinstate aqueous flow. Success is accomplished when the bleb reforms or becomes more diffuse after injecting balanced salt solution into the anterior chamber through a previously formed paracentesis. The conjunctival entry sites may be closed with suture or hand-held cautery.
A novel bleb revision technique (Figure 11): https://youtu.be/Phw9HZRyC_8
Conclusion
Trabeculectomy remains the gold standard procedure for lowering IOP in patients with glaucoma or ocular hypertension. Risk assessment, perioperative observation and management, and selection of the proper intraoperative techniques are crucial to ensuring both short-term and long-term safety and success of the procedure. Gaining a comprehensive understanding of the theories underlying the various approaches and techniques to guarded filtration surgery is a lifelong learning endeavor for those seeking to master this procedure.
Table 4-1: Additional pearls to enhance outcomes post trabeculectomy
| Short conjunctival flap (limbus-based flap) | Reduces potential filtration area and increases likelihood of postoperative failure. Initiate conjunctival flap at least 8 mm from the limbus. |
| Toothed forceps for conjunctival manipulation | Use of nontoothed forceps and gentle handling of tissues reduces buttonholes and postoperative inflammation. |
| Thin or small scleral flap | The scleral flap should be at least half the total scleral thickness and large enough to functionally cover the sclerectomy site to prevent prolonged postoperative hypotony. |
| Paracentesis too small, cannot be found or cannot be cannulated | To cannulate easily, a paracentesis must have a large internal (endothelial) opening and known location and orientation (test paracentesis before continuing case). |
| Iridectomy imperforate or too small | The iridectomy must be patent and extend to the posterior sclerectomy margins (so one can see the red reflex or ciliary processes). Extreme caution must be used to enlarge a small iridectomy to avoid vitreous loss. |
| Sclerectomy site too far posteriorly | Excessive bleeding or occlusion of the sclerectomy by the ciliary body can be avoided by making the initial anterior chamber entry site as far anterior as possible (well into the limbal gray-blue zone). |
| Sclerectomy site too close to lateral scleral flap margin | The scleral flap must completely cover the sclerectomy, otherwise resistance to aqueous flow will be low and hypotony will result. |
| Occlusion of the sclerectomy by ciliary processes | Particularly seen in small hyperopic eyes. An anterior sclerectomy site is helpful. Ciliary processes can be gently cauterized, grasped with 0.12 forceps and excised with Vannas scissors if necessary. |
| Vitreous loss through sclerectomy site | Fortunately a rare complication. A meticulous Weck-Cel vitrectomy can salvage the bleb. Postoperative hypotony with a shallow anterior chamber must be avoided (to prevent posterior vitreous from entering the sclerectomy site). |
| Scleral flap closure (too tight or too loose) | With irrigation through the paracentesis, fluid should flow slowly with a maintained anterior chamber depth. |
| Conjunctival wound leak | Wound leaks can be reduced by meticulous wound closure and testing with fluorescein. |
References
- ↑ AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 11. Risk fac-tors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134(4):481-498.
- ↑ Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10(2):112-116.
- ↑ Law, SK, Song BJ, Yu F, Kurbanyan K, Yang TA, Caprioli J. Hemorrhagic complications from glaucoma surgery in patients on anticoagulation therapy or antiplatelet therapy. Am J Ophthalmol. 2008;145(4):736-746.
- ↑ Kohl DA, Walton DS. Limbus-based versus fornix-based conjunctival flaps in trabeculectomy: 2005 update. Int Ophthalmol Clin. 2005;45(4):107-113.
- ↑ Skuta GL, Beeson CC, Higginbotham EJ, et al. Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology. 1992;99:438-444.
- ↑ Shields MB, Scroggs MW, Sloop CM, et al. Clinical and histopathologic observations concerning hypotony after trabeculectomy with adjunctive mitomycin C. Am J Ophthalmol. 1993;116:673-683.
- ↑ Rockwood EJ, Parrish RK II, Heuer DK, et al. Glaucoma filtering surgery with 5-fluorouracil. Ophthalmology. 1987;94:1071-1078.
- ↑ Kitazawa Y, Suemori-Matsushita H, Yamamoto T, et al. Low-dose and high-dose mitomycin trabeculectomy as an initial surgery in primary open-angle glaucoma. Ophthalmology. 1993;100:1624-1628.
- ↑ Neelakantan A, Rao BS, Vijaya L, et al. Effect of the concentration and duration of appli-cation of mitomycin C in trabeculectomy. Ophthalmic Surg. 1994;25:612-615.
- ↑ Megevand GS, Salmon JF, Scholtz RP, Murray AD. The effect of reducing the exposure time of mitomycin C in glaucoma filtering surgery. Ophthalmology. 1995;102:84-90.
- ↑ Derick RJ, Pasquale L, Quigley HA, Jampel H. Potential toxicity of mitomycin C. Arch Ophthalmol. 1991;109:1635.
- ↑ Seah SK, Prata JA Jr, Minckler DS, et al. Mitomycin-C concentration in human aqueous humour following trabeculectomy. Eye. 1993;7:652-655.
- ↑ Starita RJ, Fellman RL, Spaeth GL, et al. Short and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938-946.
- ↑ Schwartz B. The response of ocular pressure to corticosteroids. Int Ophthalmol Clin. 1966;6(4):929-989.
- ↑ Savage JA, Simmons RJ. Staged glaucoma filtration surgery with planned early conver-sion from scleral flap to full-thickness operation using the argon laser. Ophthalmic Laser Therapy. 1986;1:201.
- ↑ Pappa KS, Derick RJ, Weber PA, et al. Late argon laser suture lysis after mitomycin C trabeculectomy. Ophthalmology. 1993;100(8):1268-1271.
- ↑ Spaeth GL. Ophthalmic Surgery: Principles and Practice. Philadelphia, PA: Saunders; 2003.
- ↑ Shetty RK, Wartluft L, Moster MR. Slit-lamp needle revision of failed filtering blebs using high-dose mitomycin C. J Glaucoma. 2005;14(1):52-56.