Canaloplasty
| Primary authors |
|
|---|
Editors Note: This article focuses on ab externo canaloplasty. Ab interno approaches are now more common and will be covered in a future chapter.
________________________________________________________________
Traditionally, the gold standard for surgical treatment of open-angle glaucoma (OAG) has been trabeculectomy with the use of antifibrotic therapy. The goal of the trabeculectomy procedure is to create an alternative route for aqueous humor to drain out of the eye and into a subconjunctival reservoir, thus creating a bleb. Despite the fact that the trabeculectomy surgery has proven to be effective in both lowering intraocular pressure (IOP) and halting the progression of the disease process, it is not without the risk of immediate or delayed postsurgical complications.[1][2][3][4][5][6] Therefore, there has been a growing interest amongst surgeons to seek out and develop IOP-lowering procedures that do not rely on the creation of a bleb and avoid the utilization of antifibrotics. One such procedure that has successfully fulfilled these requirements is canaloplasty with circumferential dilation and suture tensioning of Schlemm’s canal. Canaloplasty has gained increasing popularity as a surgical procedure that promotes the rejuvenation of the natural conventional outflow pathway without the formation of a bleb. Studies have shown that canaloplasty has proven to be similar to trabeculectomy in effectively lowering IOP and appears to have a safer postoperative profile.[7][8]
Surgical Procedure
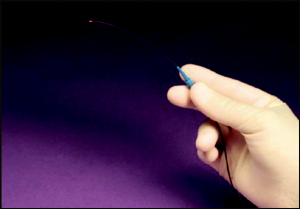
Canaloplasty is accomplished by exposing Schlemm’s canal via non-penetrating dissection and using the iTrack 250 flexible microcatheter (Figure 40 -1) to circumferentially viscodilate and intubate Schlemm’s canal with a tensioning suture. Furthermore, the microcatheter is also unique in that it has a beacon tip to allow for transscleral illumination during catheterization.
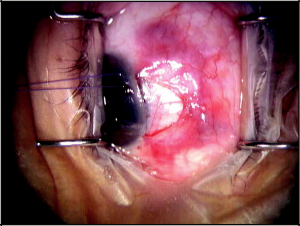
Typically, anesthesia and akinesia are achieved with a retrobulbar block. A corneal traction suture is placed superiorly next to the limbus. Next, a fornix-based conjunctival incision is created, followed by careful dissection of conjunctiva and Tenon’s capsule down to bare sclera (Figure 40-2). I favor a superonasal approach during this step, as it leaves the superior and superotemporal conjunctiva undisturbed should future incisional surgeries be necessary. Hemostasis is achieved with wetfield cautery. No antifibrotics, such as mitomycin C (MMC), are necessary.
Once bare sclera is exposed, a superficial one-third- to one-half-thickness scleral flap is created at the limbus. A 5x5mm parabolic shape may be used; however, I predominantly use a triangular scleral flap. Within the base of the superficial scleral flap, a deep scleral flap is created with a dissection plane just superficial to the choroid. A 4x4mm parabolic deep scleral flap may suffice, or a triangular deep scleral flap smaller than the superficial flap may be preferred. The choroid may be slightly visible beneath the deep scleral flap. The deep scleral flap is dissected anteriorly to unroof Schlemm’s canal.
While dissecting forward, pay close attention to identifying the cross striations of the scleral spur. This assures that the surgeon has reached the correct depth and plane while fashioning the deep flap. Identifying the cross striations of the scleral spur also anatomically orients the surgeon to the location of Schlemm’s canal, which is immediately anterior.
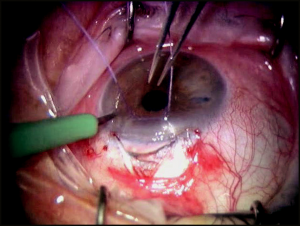
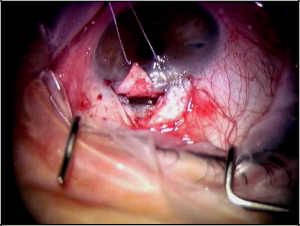
At this point, a paracentesis is performed to lower the IOP to the mid- to high-single digits (Figure 40-3). This serves to decompress the eye and decrease the risk of perforating into the anterior chamber while isolating Schlemm’s canal and creating Descemet’s window. After the canal is identified, the deep flap is carefully dissected further anteriorly to detach Schwalbe’s line and to create an appropriately sized Descemet’s window, which should, at minimum, be 500 µm (Figure 40-4). Aqueous usually, but not always, percolates through the Descemet’s window. The iTrack microcatheter is then inserted through one of the canal ostia. The lights are dimmed to allow visualization of the lighted tip of the microcatheter as it is advanced through Schlemm’s canal (Figure 40-5). If an obstruction is encountered during canulation, the microcatheter may be retracted, inserted into the opposite ostia, and cannulated in the other direction to achieve successful passage. After circumferential cannulation is completed, a 10-0 polypropylene suture is tied to the distal end of the catheter, which is retracted, introducing the suture into Schlemm’s canal. As the suture is pulled through, ophthalmic viscosurgical device is injected at a rate of 0. 5 μL/2 hours (1/8 turn of the OVD injector every 2 clock hours). The suture is tied, allowing appropriate tension to be placed on the canal without inadvertently performing a trabeculotomy. Suture tensioning is critically important because this allows for tension to be transmitted 360 degrees on Schlemm’s canal and the trabecular meshwork, thereby restoring natural aqueous outflow.
Creation of a scleral lake is accomplished by excisioning the deep scleral flap followed by watertight closure of the superficial scleral flap with interrupted 10-0 nylon sutures. The conjunctiva and Tenon’s capsule is reapproximated with 8-0 Vicryl at the limbus, and finally subconjunctival antibiotics are given along with topical antiobiotic-steroid ointment.
Conclusion
Canaloplasty offers an approach to surgically treating open-angle glaucoma without the formation of a bleb and lower chances of complications compared to trabeculectomy.[1][2][3][4][5][6][9][10][11][12][13] Ideal patient selection is key when considering canaloplasty. Patients with mild to moderate OAG with IOP desired in the mid- to low-teens are perfect candidates for canaloplasty. Furthermore, for patients with very thin conjunctiva in whom trabeculectomy with antifibrotics or glaucoma drainage device surgery would be much less desirable, canaloplasty has offered a surgical option for treating such difficult cases. Canaloplasty also appears to be an option for patients with uncontrolled ocular hypertension on maximum-tolerated medical therapy for whom laser trabeculoplasty could pose unsafe IOP spikes after laser.
Despite the increased popularity and rising acceptance of canaloplasty, like all other glaucoma surgical procedures, it has its limitations. Canaloplasty may be contraindicated in patients with chronic angle closure, narrow angles, angle recession, neovascular glaucoma, and in eyes that have undergone previous glaucoma procedures that preclude adequate cannulation of Schlemm’s canal.[14] Canaloplasty outcomes will be limited in eyes in which the distal aqueous outflow network is permanently scarred down or collapsed. A learning curve for successful catheterization of Schlemm’s canal and certain anatomical variations may prohibit successful catheterization, such as the microcatheter tip entering a large collector channel or meeting unknown resistance.[8] Perhaps, the biggest limitation of canaloplasty with suture tensioning is the lack of long-term data. However, because of current promising data,[7][8] and the aforementioned advantages of canaloplasty, this procedure has made its way into the surgical armamentarium of the glaucoma specialist.
Key Points
- Ideal patient selection includes the following:
- Mild to moderate open-angle glaucoma with IOP desired in the mid- to low-teens.
- Patients with very thin conjunctiva in which trabeculectomy with antifibrotics or glaucoma drainage devices (GDDs) would be less desirable.
- Uncontrolled ocular hypertension with impending optic nerve damage on maximum tolerated medication.
- Contraindications for canaloplasty include the following:
- Chronic angle closure.
- Narrow angles.
- Angle recession.
- Neovascular glaucoma.
- Eyes that have undergone previous glaucoma procedures that preclude adequate cannulation of Schlemm’s canal.
- Surgical technique—2 points that will promote maximal pressure-lowering response are the following:
- Appropriately sized Descemet’s window (≥500 µm).
- Adequate suture tensioning.
References
- ↑ 1.0 1.1 Jones E, Clarke J, Khaw PT. Recent advances in trabeculectomy technique. Curr Opin Ophthalmol. 2005;16:107-113.
- ↑ 2.0 2.1 Borisuth NSC, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10:112-116.
- ↑ 3.0 3.1 Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up; the Tube Versus Trabeculectomy Study Group. Am J Ophthalmol. 2007;143:23-31.
- ↑ 4.0 4.1 Scott IU, Greenfield DS, Schiffman J, et al. Outcomes of primary trabeculectomy with the use of adjunctive mitomycin. Arch Ophthalmol. 1998;116:286-291.
- ↑ 5.0 5.1 Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MW, Guire KE. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS); the Collaborative Initial Glaucoma Treatment Study Group. Am J Ophthalmol. 2005;140:16-22.
- ↑ 6.0 6.1 Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy. III. Early and late complications. Eye. 2002;16:297-303.
- ↑ 7.0 7.1 Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814-824.
- ↑ 8.0 8.1 8.2 Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract; one-year results. J Cataract Refract Surg. 2008;34:433-440.
- ↑ Mac I, Soltau JB. Glaucoma-filtering bleb infections. Curr Opin Ophthalmol. 2003;14:91-94.
- ↑ Ophir A. Encapsulated filtering bleb; a selective review–new deductions. Eye. 1992;6: 348-352.
- ↑ Bindlish R, Condon GP, Schlosser JD, D’Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy; five-year follow-up. Ophthalmology. 2002;109:1336-1341.
- ↑ Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol. 2006;90:175-180.
- ↑ King AJ, Rotchford AP, Alwitry A, Moodie J. Frequency of bleb manipulations after trabeculectomy surgery. Br J Ophthalmol. 2007;91:873-877.
- ↑ 14. Khaimi MA. Canaloplasty using iTrack 250 microcatheter with suture tensioning on Schlemm’s canal. Middle East Afr J Opthalmol. 2009;19:127-129.