Glaucoma Surgery in Corneal Transplant Patients
| Primary authors |
|
|---|
Glaucoma is a frequent comorbid condition in corneal transplant patients. Glaucoma may develop after transplantation secondary to chronic steroid use or synechiae-induced angle closure, or it could be a pre-existing condition related to traumatic injury. Common indications for corneal trans-plant include Fuchs’ dystrophy, aphakic or pseudophakic bullous keratopathy, corneal infections, traumatic injury, scarring, and irregular astigmatism. Penetrating keratoplasty performed for aphakic and pseudophakic bullous keratopathy, as well as inflammatory conditions, are more likely to cause postoperative glaucoma than keratoconus and Fuchs’ endothelial dystrophy because the former is often associated with damage to the trabecular meshwork. Newer transplantation techniques, such as Descemet’s stripping endothelial keratoplasty (DSEK), used to treat corneal edema secondary to endothelial dysfunction (ie, Fuchs’ dystrophy, aphakic and pseudophakic bullous keratopathy) have allowed for faster visual recovery. However, glaucoma is still a significant comorbid condition.
In the Collaborative Corneal Transplantation Studies, a history of preoperative glaucoma increased the graft failure rate from 29% to 48%.[1] Further, in the Cornea Donor Study, the rate of graft failure was 11% with-out glaucoma, 20% with glaucoma treated with medications alone, 29% with glaucoma treated with surgery alone, and 58% with glaucoma treated by both medication and surgery.[2] Repeated studies have shown that the risk of graft failure is increased in patients with uncontrolled intraocular pressure (IOP), making management of glaucoma an integral component to graft survival.[3][4]
Preoperative risk factors for uncontrolled IOP after corneal transplant include the following:
- Steroid-induced ocular hypertension
- Prior angle trauma
- Pre-existing glaucoma with uncontrolled IOP
- Aphakia
- Intolerance or allergy to IOP-lowering medications
- Poor compliance with medications
Surgical Options
Surgical intervention for glaucoma primarily includes either trabeculectomy with antimetabolite or implantation of a glaucoma drain-age device. In the case of refractory glaucoma, cycloablative procedures such as cyclophotocoagulation may be considered.[5] The major factors influencing one’s decision regarding these surgical options include the intended degree of IOP reduction and the risk of bleb failure due to comorbidities. Trabeculectomy may achieve a lower IOP compared with glaucoma drain-age devices (GDDs). However, trabeculectomy is more sensitive to post-operative inflammation, and when performing combined lens exchange and/or vitrectomy, there may be a higher risk for bleb failure. Further, trabeculectomy surgery may not be feasible in patients who have prior conjunctival scarring secondary to trauma, chemical exposure, limbal stem cell deficiency, or other ocular surface diseases. Thus, prior to performing trabeculectomy, it is important to have any ocular surface inflammation under maximal control.
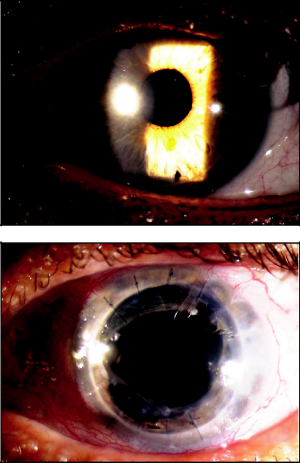
The surgical procedure of choice for glaucoma requiring moderate IOP reduction in the setting of prior corneal transplant is a GDD (Figure 35-1). Typically, these devices allow for adequate IOP control and are relatively uncomplicated to implant. Further, multiple GDDs can be placed to achieve lower IOP. Tube shunt surgery can be done at the same time as penetrating keratoplasty in a staged fashion, with the tube shunt performed 4 to 6 months prior to corneal surgery or after prior corneal transplant. Valved (Ahmed, Krupin, Molteno) and nonvalved (Baerveldt) implants can be used alone or in combination. Careful placement of the GDD within the anterior chamber is of importance, as there is a risk of endothelial damage should repeated contact occur between the tube and endothelium.
Surgical Planning
Preoperative Clinical Considerations
- Posterior capsule and hyaloid face status
- Presence or absence of vitreous into the anterior chamber
- Altered lens status: aphakia; sutured posterior chamber lens
- Ocular surface inflammation
- Conjunctival vascularity and/or scarring
A thorough preoperative examination for the presence of vitreous in the anterior chamber is essential prior to surgical intervention. The presence of vitreous in the anterior chamber increases the risk of glaucoma surgery failure. Vitreous can become incarcerated into the tube opening or sclerostomy site, causing poor outflow and elevated IOP. If vitreous is present, a thorough anterior vitrectomy is required at the time of glaucoma surgery to prevent failure.
Ocular surface inflammation and vascularity can be a source of bleb failure in patients undergoing trabeculectomy. Adequate control of inflammation with steroids or immunomodulating agents is essential prior to surgery. GDD is preferred in patients with significant ocular surface inflammation, as these patients have a tendency toward developing bleb fibrosis with subsequent bleb failure.
Anterior chamber depth is important when considering implantation of a GDD in a patient with either penetrating keratoplasty (PK) or DSEK. Eyes with a shallow anterior chamber depth are at higher risk for tube– endothelium contact, which risks failure of the transplanted tissue and corneal decompensation over time.
Intraoperative Considerations
- Location of tube placement
- Length of tube
- Placement of venting slits and/or ripcord
GDD tubes can be placed in the anterior chamber or through the pars plana. The length of the tube is of critical importance because cor-neal decompensation and graft failure will ensue with chronic tube–cornea touch. Placement of the tube in the pars plana prevents tube–cornea touch, but a complete vitrectomy is required prior to the placement of the tube in this location to prevent incarceration of the vitreous.
When placing a nonvalved GDD, such as the Baerveldt, the surgeon has the option to place venting slit incisions within the tube, in addition to occluding the tube with a nylon suture and Vicryl ligature to prevent postoperative hypotony. Venting incisions within the tube allow for early flow through the tube until a bleb has formed over the plate of the tube and the ripcord can be removed, typically 4 to 6 weeks after implantation.
Postoperative Considerations
- Complications: Hypotony, graft failure, suprachoroidal hemorrhage, malignant glaucoma
- Chronic topical steroid use and potential IOP elevation
- Choice of topical ocular hypotensive medications in patients with a corneal graft
Suprachoroidal hemorrhage is a dreaded complication of cornea transplant surgery. It is more common in patients who have pre-existing glaucoma, vascular disease, and preoperative elevated IOP. Suprachoroidal hemorrhage can occur during glaucoma surgery but is more common in corneal transplantation because the IOP is reduced to atmospheric pressure. If the choroidal pressure is elevated during this time, a suprachoroidal hemorrhage and/or expulsion of intraocular contents occurs secondary to the absence of IOP to counterbalance the choroidal pressure. For this reason, in patients undergoing corneal transplantation, it is advisable to use a Honan balloon or other device to decompress the vitreous and lower IOP at the time of surgery.
Patients with corneal transplant often need to be on chronic steroid therapy. Chronic steroid therapy can increase IOP, and the elevated IOP should be weighed against the increased risk of corneal graft rejection. Intensive topical steroids are often critical early in the postoperative period to avoid graft rejection and can be gradually tapered over time. However, graft rejection can occur at any point in time, therefore, careful attention should be paid to graft health during the tapering of topical steroids.
For patients who have had corneal transplantation and glaucoma surgery whose IOP is still not adequately controlled, beta-blockers (timolol) or alpha-agonists (brimonidine) are the medications of choice. prostaglandin analogue drugs (eg, latanoprost, bimatoprost, travoprost) should be avoided because they can increase inflammation, leading to graft rejection. Similarly, carbonic anhydrase inhibitors (eg, dorzolamide, brinzolamide) can lead to graft failure by inducing decreased endothelial cell pump function, leading to corneal edema. In the end, placement of multiple glaucoma drainage devices to achieve adequate IOP control is an option if other interventions prove inadequate, as good IOP control is critical to graft survival.
Conclusion
Corneal graft failure is a potential complication that can occur at any point in the postoperative period. Should it occur, it is critical to identify the etiology of graft failure, which may be secondary to uncontrolled IOP or due to mechanical damage from glaucoma implant hardware. Graft fail-ure due to mechanical trauma from intraocular hardware should prompt revision of the tube with repeat corneal transplant. The intraocular portion of the glaucoma drainage device can be trimmed to a shorter length or repositioned to a location that is directed further away from the cornea (ie, in the iris plane, behind the iris, or in the pars plana).
Key Points
- Glaucoma is common in patients with corneal transplantation.
- Glaucoma increases the risk of graft failure.
- Glaucoma drainage devices are usually preferred in patients with corneal transplantation.
- Ideally, prostaglandins are avoided in the setting of corneal transplantation.
References
- ↑ Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101(9):1536-1547.
- ↑ Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009:116(6):1023-1028.
- ↑ Lee RK, Fantes F. Surgical management of patients with combined glaucoma and corneal transplant surgery. Curr Opin Ophthalmol. 2003;14(2):95-99.
- ↑ Aldave AJ, Rudd JC, Cohen EJ, et al. The role of glaucoma therapy in the need for repeat penetrating keratoplasty. Cornea. 2000;19(6):772-776.
- ↑ Shah P, Lee GA, Kirwan JK, et al. Cyclodiode photocoagulation for refractory glaucoma after penetrating keratoplasty. Ophthalmology. 2001;108(11):1986-1991.