Glaucoma Surgery in the Keratoprosthesis Patient
| Primary authors |
|
|---|
Keratoprosthesis: The Glaucoma Problem
Patients with a history of keratoprosthesis present many challenges for the glaucoma specialist. An extremely high incidence of glaucoma is seen in patients who have undergone keratoprosthesis surgery. Many of these patients have pre-existing glaucoma or develop glaucoma following keratoprosthesis placement. Netland et al[1] published a retrospective case series of 55 eyes of 52 patients treated with a keratoprosthesis and found glaucoma present in the majority (64%) of eyes treated with keratoprosthesis (36% before and 28% following keratoprosthesis).
The incidence of glaucoma is already high in patients who need to undergo keratoprosthesis surgery. This includes patients with a history of severe surface disease, chemical injury, ocular cicatricial pemphigoid, status/postkeratolimbal allograft surgery, herpes keratitis, and aniridia. Glaucoma can also be exacerbated following keratoprosthesis surgery. Development of angle-closure glaucoma following keratoprosthesis surgery is common. Keratoprosthesis patients are often on chronic steroids, so this predisposes them to a steroid-induced component as well.
Currently there are 2 artificial corneas approved for use in the United States—the AlphaCor (Addition Technology, Des Plaines, Illinois) artificial cornea and the Dohlman-Doane or Boston keratoprosthesis (Massachusetts Eye & Ear Infirmary, Boston, Massachusetts). The Boston keratoprosthesis was first described in 1974 by Sayech et al[2] and was approved by the Food and Drug Administration (FDA) for use in the United States in 1992. There are 2 types, depending on the severity of ocular surface disease (Type I and Type II).
The Multicenter Boston Type 1 Keratoprosthesis Study (MBTKS), a prospective series of 141 patients from 17 centers, revealed that many of these patients do reasonably well from a visual outcome standpoint.[3] The study showed a visual acuity of greater than 20/40 in 23% of patients and better than or equal to than 20/200 in 57% of patients.
Failure of visual acuity to improve was attributed to underlying ocular disease such as advanced glaucoma, macular degeneration, or retinal detachment. This study demonstrated the importance of controlling glaucoma in this patient population.
Diagnosis and Monitoring
Intraocular pressure measurement is difficult because there is no reliable way to monitor intraocular pressure (IOP) in this patient population. measurements taken over the prosthesis will be markedly elevated, and measurements taken over the sclera are not accurate.[4] At this time, finger palpation is felt to be the best approach to estimation of the IOP. I prefer palpating over the upper lid with an index finger from each hand while the patient is looking down to get an approximation of IOP. This is a skill that can be developed by a clinician by palpating many normal eyes and comparing the estimated IOP by palpation to the IOP measured with applanation tonometry. With practice, surgeons can learn to better estimate the IOP using palpation techniques.
In these patients, glaucoma is followed using tactile IOP, visual field testing, and optic nerve examinations. Stereoscopic optic nerve examinations can be difficult secondary to retroprosthetic membrane formation, nystagmus, and limited keratoprosthesis aperture. Anterior segment optical coherence tomography may be useful in documenting stability of the device around the stem and to better characterize angle anatomy.[5]
Eventually, an IOP sensor would be the best way to monitor IOP more accurately in keratoprosthesis patients. Techniques using laser interferometry (eg, FISO Inc, Ottawa, Canada) and Radiowave telemetry (eg, Medical Sensors Technology Inc, Germany) may hold some promise.[6] The future application of these technologies in humans is yet to be determined.
Management
Medications
Medications can be helpful, but many patients require surgical intervention. Typically, aqueous suppressants are a good option for first-line treat-ment. Prostaglandin analogues can also be helpful in these patients. preservative-free preparations, such as preservative-free timolol maleate, can be an ideal option in keratoprosthesis patients with severe surface disease. These can minimize the toxic surface effects of preservatives, as many patients with a keratoprosthesis have severe underlying surface disease. Oral carbonic anhydrase inhibitors, such as acetazolamide or methazolamide, can also be helpful in keratoprosthesis patients when the IOP is difficult to control.
Glaucoma Drainage Implants
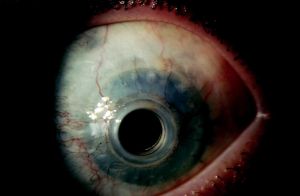
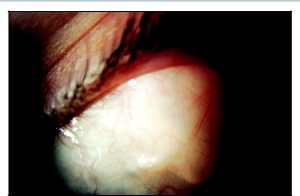
Various options of glaucoma drainage implants can be used in these patients, but I prefer either an Ahmed or smaller-plate Baerveldt (250 mm2). A Molteno3 (175 mm2) would also be a good option in these patients because of the theoretical lower risk of hypotony. Tube placement should be done prior to keratoprosthesis placement when possible. Drainage implants placed in the anterior chamber prior to keratoprosthesis placement usually continue to function well following keratoprosthesis surgery. There is a risk of tube occlusion from retroprosthetic inflammatory membrane formation in tubes placed in the anterior chamber prior to keratoprosthesis placement. If the patient has already had keratoprosthesis surgery and develops glaucoma following surgery, a combined pars plana vitrectomy with pars plana tube insertion is a good option (Figures 36-1 and 36-2). I recommend doing this in conjunction with a vitreoretinal surgeon who is comfortable with endoscopic vitrectomy techniques because the view can be limited and the patient is at risk of tube occlusion by vitreous without a complete vitrectomy. Glaucoma drainage implant surgery can also be done at the time of keratoprosthesis placement but may be difficult to arrange for logistical reasons.
A recent published report described a series in which the glaucoma drainage implant plate was connected with a tube to the lacrimal sac, ethmoid or maxillary sinus, or lower lid fornix to facilitate aqueous drainage in keratoprosthesis patients.[7] The authors found that the incidence of severe infection rates was very low with these techniques. Although this technique is reasonable, more conventional tube shunt surgery has been shown to be effective in keratoprosthesis patients. Vajaranant et al[8] recently published a cases series using the technique of combined vitrectomy and pars plana glaucoma shunt placement in keratoprosthesis patients.
Cyclophotocoagulation
I prefer the transscleral cyclophotocoagulation option in patients with severe conjunctival scarring who are poor candidates for tube shunt placement. Recently, Rivier and colleagues reported a series between 1993 and 2007 in which 18 eyes of 18 patients underwent diode laser transcleral cyclophotocoagulation (DLTSC), either before (n = 3), during (n = 1), or after (n = 14) keratoprosthesis surgery. Mean postoperative IOP was significantly reduced at 6, 12, 24, 36, and 48 months after DLTSC.[9] In my practice, I typically begin with 180 degrees of treatment, which is usually adequate in achieving the desired IOP lowering in most patients. Because cyclodestructive procedures are permanent, I recommend conservative treatment initially, as more treatment can always be added in the future.
Endoscopic cyclophotocoagulation is a good option in patients who have severe surface disease, conjunctival scarring, or prior keratolimbal allograft surgery. In my experience, transscleral cyclophotocoagulation may not be as effective following keratolimbal allograft surgery given the increased thickness of perilimbal tissue that needs to be penetrated to reach the ciliary processes. In patients with severe conjunctival scarring that precludes tube shunt placement, endoscopic cyclophotocoagulation can be a great option if a vitrectomy is already being performed for another indication.
Key Points
- Patients with a history of keratoprosthesis surgery have a high incidence of glaucoma and need to be followed very closely to prevent permanent visual loss.
- Medications, aqueous drainage implants, and cyclodestructive procedures are all reasonable options, but many patients will require surgical intervention.
- Be ready for additional challenges when caring for these patients.
- Better ways of measuring IOP are needed in these patients to improve long-term monitoring capabilities. New technologies may eventually make this possible.
References
- ↑ Netland, PA, Terada H, Dohlman, CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105(4):751-757.
- ↑ Sayech RR, Ang LPK, Foster CS, Dohlman CH. The Boston keratoprosthesis in Steven’s-Johnson syndrome. Am J Ophthalmol. 2008;145(3):438-444.
- ↑ Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113(10):1779.e1-7.
- ↑ Birkholz ES, Goins KM. Boston keratoprosthesis: an option for patients with multiple failed corneal grafts. Accessed March 9, 2009. Retrieved from www.webeye.ophth.uiowa. edu/eyeforum/cases/94-Boston-Keratoprosthesis-Failed-Corneal-Grafts.htm.
- ↑ Garcia JP, de la Cruz J, Rosen RB, Buxton DF. Imaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopy. Cornea. 2008;27(2):180-188.
- ↑ Melki S, Lopez M, Dohlman C. Intraocular pressure sensors in KPRO. Boston Keratoprosthesis Update, Newsletter VI: 2009. Accessed February 10, 2010. Retrieved from www.masseyeandear.org/gedownload!/2009%20KPro%20Newsletter.pdf?item_ id=48224001&version_id=48224002.
- ↑ Dohlman CH, Grosskreutz CL, Chen TC, et al. Shunts to divert aqueous humor to distant epithelialized cavities after keratoprosthesis surgery. J Glaucoma. 2010:19(2):111-115.
- ↑ Vajaranant TS, Blair MP, McMahaon T, Wilensky JT, de la Cruz J. Special considerations for pars plana tube-shunt placement in Boston type 1 keratoprosthesis. Arch Ophthalmol. 2010;128(11):1480-1482.
- ↑ Rivier D, Jayter P, Kim E, Dohlman, CH, Grosskreutz CL. Glaucoma and keratopros-thesis surgery: role of adjunctive cyclophotocoagulation. J Glaucoma. 2009;18(4): 321-324.