Transscleral Cyclophotocoagulation
| Primary authors |
|
|---|
Full Lecture: https://www.youtube.com/watch?v=STPWKSlNa9w
Transscleral cyclophotocoagulation (TSCPC) is a laser procedure to reduce intraocular pressure (IOP) by decreasing the production of aqueous humor. The surgeon applies laser energy through the conjunctiva, Tenon’s capsule and sclera with the goal to coagulate the tissue of the ciliary body and ciliary processes. After treatment, reduced aqueous humor inflow lowers fluid load to poorly functioning outflow pathways, similar to the effect of various medications (eg, beta-blockers). Response depends on the treatment method and amount of pretreatment aqueous humor outflow impediment.
More recently, a new approach to CPC has been introduced which leverages micropulse technology rather than the traditional continuous wave (CW) method of energy delivery. Micropulse Cyclophotocoagulation (MP-CPC, Iridex Corp, Mountain View, California) is intended for reduction of aqueous humor secretion from the ciliary body epithelium of the ciliary body but may also increase aqueous outflow by placing the trabecular meshwork under stretch secondary to ciliary body tissue modulation.
Indications and Contraindications
Refractory glaucoma is the usual indication for TSCPC.[1] This includes failures of previous filtering surgery, eyes with limited vision potential and excessively high IOP despite maximum acceptable medical treatment, painful glaucomatous eyes with both highly elevated IOP and little or no vision potential, eyes with severe surface scarring precluding additional filtration procedures, or eyes with recent onset (open-angle stage) of neovascular glaucoma. TSCPC may be helpful in eyes with refractory glaucoma after penetrating keratoplasty, uveitic glaucoma, intravitreal silicone oil, refractory pediatric glaucoma, and failure of previous tube-shunt surgery. Patients with medical contraindications or who refuse invasive surgery may be able to have TSCPC.
Eyes with recalcitrant glaucoma are often at risk of imminent loss of vision from glaucoma. Eyes with good vision are not excluded, but approximately 25% of eyes with serious glaucoma problems have postoperative vision worse than the pretreatment level, whereas some eyes have slightly improved vision.[2]
TSCPC is not for eyes with total occlusion of outflow, as complete cessation of inflow would be required for IOP to be reduced to acceptable levels, and poor IOP reduction would likely result.
TSCPC is often performed in an office setting or a minor surgery facility. The procedure usually requires retrobulbar anesthesia, but it can be done with general anesthesia.
Benefits
The surgeon can perform TSCPC expeditiously in urgent situations. TSCPC does not preclude alternative procedures if it fails. Reported success of the first step of TSCPC after 1 to 2 years varies from 50% to more than 90%.[1][3]
Risks
Postoperative pain is common during the first 48 hours. Rarely, there is hyphema or fibrinous reaction. TSCPC is not incisional, so risk of postoperative intraocular infection is absent. The procedure may fail to control the IOP elevation sufficiently, requiring a second or third step of TSCPC. The risk of cystoid macular edema and loss of vision varies depending on the treatment population and preoperative characteristics and this aspect of the post-operative course should be explained in detail to appropriately inform the patient.
Surgery
Preparation
Patients should be aware of the plan, requirements, benefits, risks, and alternatives; all questions should be addressed. Warn patients about potential for postoperative pain, possible reduced vision, and possibility of need for more than one step of treatment to achieve control. Surgery is done with infrared, 810-nm diode laser irradiation delivered with a fiber optic handpiece (G-Probe, Iridex Corp, Mountain View, California). The surgeon is responsible for checking the system to ensure readiness for use prior to anesthesia.
Anesthesia
Local peribulbar and retrobulbar anesthesia is the usual method. TSCPC requires a thorough block; lesser amounts of anesthesia are seldom sufficient. Gentle touching of the perilimbal conjunctiva with forceps allows testing for sufficient numbness.
| Iris Color | Power (W) | Duration (s) | Energy Per Application (J) |
|---|---|---|---|
| Brown | 1.25 | 4.0 to 4.5 | 5.0 to 5.6 |
| All others | 1.5 | 3.5 to 4.0 | 5.25 to 6.0 |
Methods
For TSCPC, the common teaching is to start with 1.75-W power and 2.0 -s duration (3.5 J per application); adjust power downward or upward in 0.25-W increments according to whether there are excessive tissue “pops” (Figure 1, evidence of boiling of intracellular water and aqueous) during applications. Eyes with darker pigmentation require slightly lower power and energy per application to obtain equivalent results. With this technique, occasional “pops” will occur. If they occur with every application, the power is too high and should be reduced. Consider lengthening the duration of the laser applications at lower power.
A growing number of ophthalmologist are utilizing a "low and slow" approach to CPC. The "low" refers to lower energy of 1-1.5W and the "slow" refers to longer application times of 3-4seconds. For eyes with dark and medium brown iris color, use 1.25-W power and 4.0- to 4.5 -s duration (5.0 to 5.6 J per application). For eyes with lesser degrees of iris pigmentation (light brown, hazel, or blue) use 1.5-W power and 3.5- to 4.0-s duration (5.25 to 6.0 J per application) (Table 1).
Unlike traditional TSCPC, MP-CPC delivers short bursts of energy (ex. 0.5 ms) followed by rest periods (ex 1.1 ms). This approach has been described as the “duty cycle” and refers to the percentage of time during which the laser is delivering energy. Data comparing traditional TSCPC to MP-CPC are sparse and evolving.
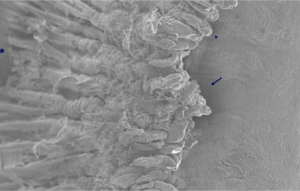
Details of Treatment
Eye Protection
The G-Probe is ready for use on opening its sterile package. Cleaning is not necessary; however, if it becomes contaminated with tissue debris during the procedure, clean the G-Probe tip. Discard the probe if debris or discoloration on the tip is not removed by cleaning. Conjunctival or scleral burns are not typical and indicate contamination at the tip.[4] Retinal irradiation due to laser transmission during TSCPC with the G-Probe is well within safety guidelines.
Keep the eye surface moist during treatment to enhance probe-tip-to-eye mating. This is especially true if the surgeon uses a lid retractor that inhibits blinking.
Delivery
Treat 3-4 quadrants, making approximately 6 applications per quadrant. Some surgeons may choose to omit one quadrant at time of initial treatment to avoid hypotony in cases that may be predisposed (Figure 2).
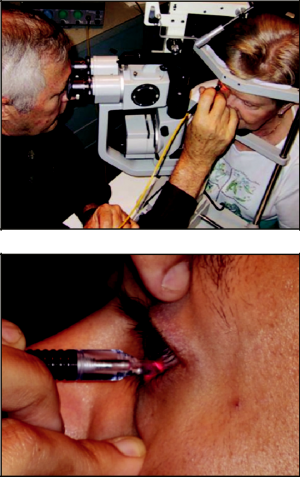
Some surgeons choose to use a slit lamp biomicroscope (Figure 3) for better placement of the G-probe. However, most surgeons today choose to perform TS-CPC in the operating room while the patient is supine and without use of a microscope.
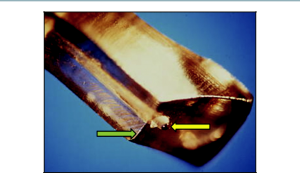
The location and spacing of applications are guided by the footplate of the G-Probe (Figures 3 and 4). The protruding fiber optic tip is hemispherical in shape. It indents conjunctiva, Tenon’s, and sclera during treatment, increasing transmission of energy to the targeted tissue.
During applications, the footplate is positioned with the curved anterior edge on the anterior border of the limbus. Each application blanches 3 to 4 ciliary processes. Each subsequent application is spaced one-half width of the footplate. The rounded fiber optic tip, protruding 0.7 mm from the footplate, causes a slight indentation at each treatment site. This is a marker for the next application (ie, during the next application, the trailing edge of the footplate bisects the indention at the site of the previous application). The video at the top of this page goes into more detail for MP-CPC energy delivery and unique probe features.
Postoperative Management
Apply a strong, long-lasting cycloplegic (Atropine 1%) and a topical steroid after the procedure and patch the eye. Continue cycloplegic 2-4 daily and steroid drops 3 or 4 times daily for at least 2 weeks and longer if required (titrated according to amount of inflammation observed).
Potential Postoperative Problems
Change of Visual Acuity
Decrease of 2 or more Snellen lines has been reported in various studies in anywhere from 12% to 40% of eyes treated with TSCPC (mean: approximately 25%).[2][3] Eyes with pre-existing poor vision appear more likely to experience a decrease in visual function. The decrease of vision, which sometimes improves with healing, has to be considered compared with the expected deterioration that would occur in the absence of intervention. Although it is seldom discussed, improved vision has also been observed after TSCPC.[1][2] Data on vision post MP-CPC are not widely available at this time (see video at top of page for further details).
Sympathetic Ophthalmia
This serious problem has occurred in a small number of fellow eyes after ciliary destructive procedures.[5] According to anecdotal reports, one case followed diode laser TSCPC in a child and another occurred in an adult.
Phthisis
In one series of 206 eyes, phthisis occurred in 4 (1.9%) eyes after traditional CW CPC.[2] There are anecdotal reports of additional cases, usually in eyes with severe problems.
Outcomes
The IOP will often dip during the first weeks. Often, as inflammation clears, IOP slowly rises to a new plateau lower than the pretreatment level. Starting after approximately 6 weeks, provided a substantial decrease of IOP has occurred and has been maintained, medications for glaucoma may be reduced while monitoring to assure IOP stays within target. Patients are often able to reduce slightly topical or systemic medical glaucoma treatment after both traditional TSCPC and MP-CPC, yet most eyes continue to need some medical treatment.
References
- ↑ 1.0 1.1 1.2 Kosoko O, Gaasterland DE, Pollack IP, Enger CL; The Diode Laser Ciliary Ablation Study Group. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. Ophthalmology. 1996;103(8):1294-1202.
- ↑ 2.0 2.1 2.2 2.3 Wilensky JT, Kammer J. Long-term visual outcome of transscleral laser cyclotherapy I: eyes with ambulatory vision. Ophthalmology. 2008;111(7):1389-1392.
- ↑ 3.0 3.1 Pastor SA, Singh K, Lee DA, et al. Cyclophotocoagulation. A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108(11):2130-2138.
- ↑ Gaasterland D, Pollack I. Initial experience with a new method of laser transscleral cyclophotocoagulation for ciliary ablation in severe glaucoma. Trans Am Ophthalmol Soc. 1992;90:225-246.
- ↑ Bechrakis NE, Müller-Stolzenburg NE, Helbig H, et al. Sympathetic ophthalmia follow-ing laser cyclocoagulation. Arch Ophthalmol. 1994;112(1):80-84.