Drainage of Choroidal Effusions
| Primary authors |
|
|---|
Serous choroidal detachment is characterized by exudative detachment of the retina and choroid following leakage of fluid from the choriocapillaris into the suprachoroidal space. This accumulation of fluid has been known to be a complication of various intraocular surgeries (cataract, glaucoma, and retinal detachment) where hypotony is combined with postoperative inflammation.[1] The terms edema, effusion, and detachment are often used interchangeably in describing this uveal disorder.
Anatomical Features
The suprachoroidal space forms a transition zone and potential space between the choroid and the sclera and is composed of thin connective tissue fibers that branch and connect melanocytes, smooth muscle cells, and ganglion cells. Because there are virtually no capillaries or lymphatic spaces to facilitate drain-off, fluid in this area must re-enter vascular channels in the choroid and exit by way of the vortex veins[2] or seep through perforations in the sclera.[3]
Pathophysiology
The physiologic pressure in the suprachoroidal space is approximately 2 mm Hg less than the intraocular pressure (IOP) in the anterior and vitreous chambers.[4] Any decrease in the IOP is transmitted to the choroid, and the reduced pressure may promote vascular engorgement and transudation. Such mechanical factors partly explain the suprachoroidal edema that results when the pressure in the eye decreases substantially at the time of surgery or trauma.[4][5] An increase in the permeability of the choroidal vessels also increases the protein leakage into the suprachoroidal space. This reduces the intravascular colloid osmotic force that is responsible for fluid reabsorption. Relative hypotony accompanies choroidal effusion. In the past, this was attributed to decreased production of aqueous.[6] experimental evidence[7] suggests that there is increased uveoscleral outflow in eyes with choroidal detachment. Less commonly, a hemorrhagic choroidal detachment can develop from rupture of the capillary membrane.[8]
Clinical Features
In the clinical setting of ocular surgery, trauma, or inflammation, shallowing of the anterior chamber with a drop in intraocular pressure should suggest possible choroidal effusion. With forward displacement of the lens–iris diaphragm and angle closure, the IOP may be elevated.[9] Acute onset of myopia, resulting from the anterior displacement of the lens, may also be a clue.[10] Visual acuity usually is reduced, including light perception, depending on the degree of interference with the visual axis. Choroidal edema may resemble retinal detachment; however, darkness of the uvea, lack of tremulousness, and normal retinal vessels indicate a probable uveal process. Visualization of the ora serrata without scleral depression is also a reliable sign.[11] The onset of a hemorrhagic choroidal detachment is usually accompanied by pain, IOP elevation, and a shallow or flat anterior chamber. Detachment can occur after a Valsalva maneuver, straining at stools, coughing, or sneezing. Anticoagulants and aspirin may facilitate bleeding. Intraoperative hemorrhage is characterized by the development of positive pressure, visualization of an enlarging dark mass obscuring the fundus reflex, and tendency to extrude eye contents.
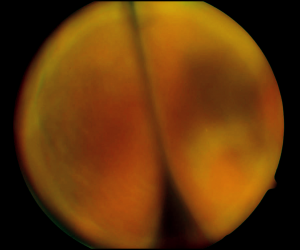
Choroidal effusions may have an annular, lobular (Figure 29-1), or flat ophthalmoscopic appearance. Annular detachments occur around the ciliary body and peripheral choroid. Lobular types are large hemispheric detachments that bulge toward the center of the globe. Flat effusions are most often apparent in isolated peripheral choroidal areas where local structures limit fluid extension. B-scan ultrasonography may be used to help differentiate between a retinal detachment and an effusion as well as between a hemorrhagic and a serous choroidal effusion. Retinal detachments are mobile and highly reflective, whereas choroidal detachments are smooth, dome-shaped, and thick. Virtually no movement of the detachment is seen with eye movement. When extensive, one can see multiple dome-shaped detachments, which may “kiss” in the central vitreous cavity. When choroidal detachments are hemorrhagic rather than serous, the suprachoroidal space is filled with a multitude of dots in contrast to the echolucent suprachoroidal space of a serous choroidal detachment.
Serous choroidal detachments usually resolve spontaneously with the normal rise in IOP that occurs during the first days to weeks postoperatively. However, prolonged edema may lead to major complications. peripheral anterior synechiae and secondary glaucoma may result from long-term flattening of the anterior chamber, especially when there is a concurrent low-grade uveitis. Secondary cataract and cyclitic membrane may develop as well. Retinal adhesions from the apposition of “kissing choroidals” and secondary retinal detachment have also been reported.[12] Persistent choroidal effusion and hypotony eventually result in phthisis bulbi.
Medical Therapy
Topical corticosteroids, cycloplegics, and mydriatics should be prescribed for patients. Oral steroids can be used and are indicated when inflammation is a factor. When the IOP is high, which can occur with haemorrhagic choroidal detachments, IOP-lowering drugs can be used. Osmotics and aqueous suppressants are recommended.
Parasympathomimetics are contraindicated. If a significant choroidal detachment persists longer than 1 week after the underlying cause has been identified and addressed, drainage of the suprachoroidal fluid should be considered. The 7-day limit is an indication only; individualized assessment is key. If an improvement is suspected, waiting longer and closely monitoring the patient may be warranted. Immediate action is indicated when lens–cornea touch or intraocular lens (IOL)–cornea touch exists. This condition causes endothelial corneal damage and acceleration of lens opacities. If the anterior chamber (AC) remains flat after the cause has been
identified and addressed, injection of viscoelastics into the AC should be considered. If lens–cornea touch or IOL–cornea touch exists, the AC reformation should be performed immediately at the slit lamp, if possible, while waiting to assess the need for suprachoroidal fluid drainage. A waiting period of 7 to 10 days after suprachoroidal hemorrhage is advised before surgical intervention to allow the fibrinolytic response to liquefy the clot, which permits more effective evacuation of the suprachoroidal space, with retinal and choroidal flattening.[13]
Surgical Therapy
The most definitive treatment of choroidal detachment is drainage of the fluid in the suprachoroidal space. If the eye is extremely soft, a sub-Tenon’s block is favored over a retrobulbar block. If the original paracentesis cannot be found, a new beveled paracentesis is made slowly with a 15-degree knife. Care in making the incision prevents rapid entry into the anterior chamber, which could result in damage to the iris or lens. If fixation of the globe becomes difficult, it may be helpful to place a small, partial-thickness scratch incision into the cornea near the limbus with a 15- degree knife. An incision approximately 2 mm long and one-third thickness is usually adequate. This groove can then be used for fixation allowing development of a paracentesis. After the paracentesis is made, the anterior chamber is reformed with injection of balanced salt solution through a 27-gauge cannula.
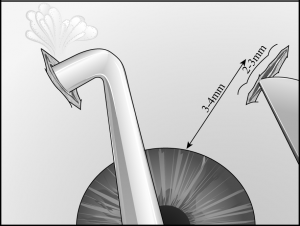
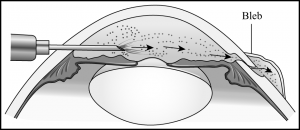
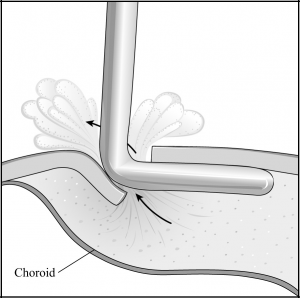
A 3- to 4-mm circumferential or radial conjunctival incision is made 3 to 6 mm from the limbus in the inferonasal and inferotemporal quadrants (away from the filtering site). Small episcleral blood vessels are gently cauterized before the sclerotomy is performed. Two 2- to 3-mm radial sclerotomies are centered in the inferior quadrants 3 to 4 mm from the limbus (Figure 29-2). We prefer to use a No. 64 Beaver blade (Becton Dickinson, Franklin Lakes, New Jersey) to make the sclerotomies because it is sharp enough to incise the sclera, but not too sharp to cut through to the choroid on contact. The blade is used to make gentle “scratch-down” strokes to approach the suprachoroidal space. Xanthochromic fluid often drains spontaneously after the incision is carried into the suprachoroidal space. Egress of fluid can be enhanced by placement of an infusion cannula through the paracentesis to pressurize the globe during these maneuvers (Figure 29-3). To further facilitate drainage, forceps are used on either edge of the sclerotomy to gape the wound further by alternatively pushing down on one edge of the sclerotomy while pulling up on the other edge of the sclerotomy. After the fluid flow slows, the tip of a cyclodialysis spatula can be carefully inserted into the suprachoroidal space in a circumferential direction (Figure 29-4). This maneuver is particularly helpful with facilitating drainage of loculated fluid as is often found in patients with hemorrhagic choroidals. The spatula is then removed and reinserted in the opposite direction. The sclerotomy is manipulated until no further fluid presents at the wound (typically, the brown color of the underlying choroid comes into view). An indirect ophthalmoscope is then used to confirm flattening of the retina before the other inferior sclerotomy is entered.
After the choroidal effusions have been drained, the edges of the sclerotomies are left unopposed to allow for continued drainage.[14] This can be accomplished by gaping the edges of the scleral wound with cautery or by excising a small piece of sclera with a punch or trephine.[15] The conjunctival wounds are closed with 8-0 Vicryl suture and atropine and antibiotic ointment are applied to the eye, followed by a patch and an eye shield. Occasionally, concurrent bleb revision is necessary and if cataract extraction needs to be performed, it may be combined with choroidal drainage.[16] The cataract surgery follows the choroidal drainage.
Key Points
- In the setting of ocular surgery, trauma, or inflammation, shallowing of the anterior chamber with a decrease in IOP suggests a possible choroidal effusion.
- Medical therapy for choroidal effusions includes topical corticosteroids, cycloplegics, mydriatics, and occasionally oral steroids.
- The most definitive treatment of choroidal effusions is surgical drainage of the fluid in the suprachoroidal space.
- Surgical drainage involves creation of two 2- to 3-mm radial sclerotomies centered in the inferior quadrants 3 to 4 mm from the limbus.
- During surgical drainage of choroidal effusions, egress of fluid can be enhanced by placement of an infusion cannula through the paracentesis to pressurize the globe.
- The sclerotomies are manipulated with forceps and a cyclodialysis spatula until no further fluid presents at the wound and the brown color of the underlying choroid comes into view.
References
- ↑ Brubaker RF, Pederson JE. Ciliochoroidal detachment. Surv Ophthalmol. 1983;27(5): 281-289.
- ↑ Weiter JJ, Ernest JT. Anatomy of the choroidal vasculature. Am J Ophthalmol. 1974;78(4):583-590.
- ↑ Bill A. Intraocular pressure and blood flow through the uvea. Arch Ophthalmol. 1962;67:336-348.
- ↑ 4.0 4.1 Moses RA. Detachment of the ciliary body: anatomic and physical considerations. Invest Ophthalmol. 1965;4(5):935-941.
- ↑ Capper SA, Leopold IH. Mechanism of serous choroidal detachment. Arch Ophthalmol. 1956;55(1):101-113.
- ↑ Chandler PA, Maumenee AE. A major cause of hypotony. Am J Ophthalmol. 1961;52: 609-618.
- ↑ Pederson JE, Gasterland DE, McClean HM. Experimental ciliochoroidal detach-ment: effect on intraocular pressure and aqueous humor flow. Arch Ophthalmol. 1979;97(3):536-541.
- ↑ Bellows AR, Chylack LT, Hutchinson BT. Choroidal detachment: clinical manifestation, therapy and mechanism of formation. Ophthalmology. 1981;88(11):1107-1115.
- ↑ Schepens CL, Brockhurst RJ. Uveal effusion: I. Clinical picture. Arch Ophthalmol. 1963;70:189-201.
- ↑ Hyman BN, Hagler WS. Bilateral annular detachment and myopia. Am J Ophthalmol. 1970;70(5):853-855.
- ↑ Hertz V. Choroidal detachment with notes on sclera depression and pigmented streaks in the retina. Acta Ophthalmol. 1954;41(Suppl):1-256.
- ↑ Berrocal J. Adhesion of the retina secondary to large choroidal detachment as a cause of failure in retinal detachment surgery. Mod Probl Ophthalmol. 1979;20:51-52.
- ↑ Lambrou FH, Meredith TA, Kaplan HG. Secondary surgical management of expulsive choroidal hemorrhage. Arch Ophthalmol. 1987;105(9):1195-1198.
- ↑ Abrams GW, Thomas MA, Williams GA, et al. Management of postoperative suprachoroidal hemorrhage with continuous infusion air pump. Arch Ophthalmol. 1986;104(10):1455-1458.
- ↑ Dellaporta A. Scleral trephination for subchoroidal effusion. Arch Ophthalmol. 1983;101(12):1917-1919.
- ↑ Berke SJ, Bellows AR, Shingleton BJ, et al. Chronic and recurrent choroidal detachment after glaucoma filtering surgery. Ophthalmology. 1987;94(2):154-162.