Repair of Cyclodialysis Clefts
| Primary authors |
|
|---|
Unique Mystery Case: https://youtu.be/vyWFWkHaauo
Ab Interno Cyclopexy: https://youtu.be/akY2BA25g8M
A cyclodialysis cleft is a separation of the circumferential insertion of the meridional ciliary muscle fibers from the scleral spur. It often occurs as a result of blunt or penetrating trauma to the eye.[1] It may also develop as a complication of intraocular surgery, usually through unintentional manipulation of the iris. Inadvertent excision of a block of scleral spur tissue during filtration surgery can also result in a cyclodialysis cleft.[2] Opening of a cleft many years after the original trauma has been described following phacoemulsification, resulting in unexplained hypotony after seemingly uncomplicated surgery.[3] A cleft may also be created when a scleral tunnel is made too deep, or when an anterior chamber intraocular lens (IOL) implant of inappropriate size is used.[2][4] Cyclodialysis has also been used as a surgical procedure for aphakic glaucoma.[5]
Epidemiology
Unintentional cyclodialysis clefts have generally been thought of as being quite rare. The Erlangen Ocular Contusion Registry reported an incidence of 3.4% in patients presenting with ocular contusion or globe rupture.[6] An incidence of 2.6% has been reported in patients undergoing trabeculotomy ab externo for developmental glaucoma.[7] However, a prospective study of 92 patients presenting with closed globe injuries in which every patient underwent ultrasound biomicroscopy (UBM) found a 35% incidence of cyclodialysis clefts in those who did not develop traumatic glaucoma and an 18% incidence in those who did develop traumatic glaucoma.[8] However, not all cyclodialysis clefts result in hypotony. In a large series of cyclodialysis procedures performed for glaucoma, there was a 9% incidence of hypotony in 291 cases.[9] Cyclodialysis procedures are rarely performed today, and so cyclodialysis clefts presently occur more often as a result of trauma than surgery. In a retrospective review of 29 patients who underwent consecutive direct surgical cyclopexy during a 13 -year time period, 26 clefts were the result of traumatic injury and 3 were postsurgical.[10]
Signs and Symptoms
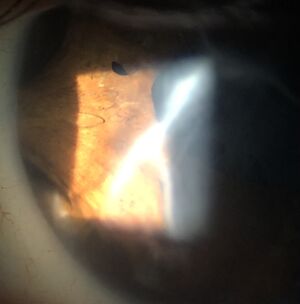
The presence of a cyclodialysis cleft should be suspected in any eye with hypotony following recent surgery or trauma after all other causes have been ruled out.[11] Other common causes of postoperative hypotony include wound leaks, overfiltering blebs, choroidal effusions, retinal detachment, and aqueous suppression due to active inflammation or prior use of aqueous suppressants.[2] Free communication between the anterior chamber and the suprachoroidal space is created by the cyclodialysis cleft, often resulting in hypotony. Associated iris injuries are not uncommon. (Figure 1) The intraocular pressure (IOP) frequently is quite low (<5 mm Hg), resulting in secondary complications such as shallow anterior chamber, induced hyperopia, cataract progression, choroidal effusion, optic disc edema, and hypotony maculopathy.[1] If untreated, it may result in permanent vision loss or phthisis. The incidence of hypotony is dependent on the flow rate between the anterior chamber and the suprachoroidal space, which is unrelated to the size of the cyclodialysis cleft.[3] Typical presenting symptoms include eye pain, tenderness, and blurred vision.[12]
Diagnosis
Various techniques may be used to identify a cyclodialysis cleft. It is important to identify the full extent of the cyclodialysis cleft and the total number of clefts to achieve complete repair of the cleft or clefts.
Gonioscopy
Using gonioscopy, the cleft appears as a deep-angle recess with a gap between the scleral spur and the ciliary body.[13] Identification of cyclodialysis clefts by gonioscopy can be challenging due to the often concomitant presence of corneal edema, hyphema, peripheral anterior synechiae, and shallow anterior chamber, as well as the softness of the hypotonous eye. Intracameral viscoelastic can improve angle visualization by making the eye firm and deepening the anterior chamber angle. Pilocarpine may also be added to induce miosis and maximally open the anterior chamber angle and cleft. However, visualization may still be poor due to corneal edema. Further, one may wish to avoid such maneuvers in the setting of hyphema by using noncontact diagnostic techniques instead.[1]
Ultrasound Biomicroscopy
UBM can provide a detailed representation of the anterior chamber, iridotrabecular angle, and ciliary body. It has been shown to be highly accurate in detecting cyclodialysis clefts, as well as anterior suprachoroidal effusions.[1] However, it can be difficult to perform in a hypotonous, tender eye.
Anterior Segment Optical Coherence Tomography
This technique has been shown to be accurate and reproducible, correlating well with UBM in the assessment of angle configuration. It has the advantage of being a noncontact technique and having higher resolution than UBM.[14] However, it does not provide visualization of the ciliary body, and thus it may miss some clefts.[1]
Scleral Transillumination
By transilluminating the sclera, a cyclodialysis cleft may be detected as a transillumination defect at the periphery of the iris.[1]
Management
Medical
Patients with cyclodialysis clefts may initially be managed conservatively, with atropine 1% twice daily to encourage apposition of the ciliary body to the sclera. Cycloplegia also deepens the anterior chamber and reduces the patient’s discomfort. It has also been suggested that inflammation may be beneficial in promoting adhesion at the cleft site, and so reduction of postoperative steroids may be considered. Filling the anterior chamber with viscoelastic may also help break the vicious cycle of hypotony.[2] However, if medical management fails to close the cleft after 6 to 8 weeks, then more aggressive measures should be considered.
Transscleral Diathermy
In this technique, the pupil is dilated and a partial-thickness scleral flap is made in the area overlying the cleft. Diathermy is then applied in the bed of the flap in the vicinity of the cleft. This induces a localized thermal burn and secondary inflammation, encouraging closure of the cleft. Both scleral ectasia and lens damage have been reported, and no more than 4 clock hours should be treated to prevent these complications.[1][12] Adequate anesthesia with a retrobulbar or sub-Tenon’s block should be achieved prior to perform-ing this technique.
Argon Laser Photocoagulation
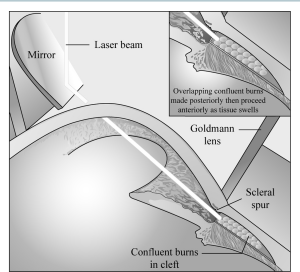
This technique, unlike the other nonincisional methods, requires an adequate view of the angle structures, without significant corneal edema or hyphema. Nevertheless, it is generally considered the first line of therapy when medical management fails. Topical anesthesia is applied, and a Goldmann 3-mirror lens is placed on the ocular surface with a coupling agent. Rows of heavy confluent burns should be placed on the scleral aspect of the cleft first, from the scleral spur toward the depths of the cleft. Then the uveal aspect of the cleft (ie, the undersurface of the ciliary body and choroid) should be treated, starting at the depth of the cleft and working anteriorly to avoid an obscured view due to uveal edema and pigment dispersion (Figure 2).[2] Recommended laser settings are as follows:
- Duration of 0.1 sec.[2][12]
- 50 to 100 µm spot size.[2][12]
- 500 to 1500 mW power.[12] Higher powers (1000 to 3000 mW) may be used on the sclera, and lower powers (800 to 1200 mW) should be used on the uvea.[2]
- Approximately 100 applications should be applied.[12]
Pretreatment with pilocarpine and modest hyperinflation of the anterior chamber with viscoelastic may be used to improve the view into the cleft. However, care must be taken to remove the viscoelastic material after the laser procedure to blunt the IOP elevation that may occur immediately after the procedure.[2] Atropine 1% is continued after treatment. If the initial treatment is unsuccessful, it may be repeated.[12] At higher powers, retrobulbar anesthesia is often needed.[15]
Cryotherapy
Under retrobulbar anesthesia, a curved retinal probe with a diameter of 2.8 mm (CR 3010, Mira Inc, Uxbridge, Massachusetts) is applied transconjunctivally 3 mm posterior to the limbus at the presumed location of the cyclodialysis cleft. Five overlapping applications, each with a duration of 30 seconds and a temperature of –85°C, are applied. atropine 1% is administered postoperatively.[16] Cryotherapy is useful in the management of smaller clefts when medical management has failed. It is advantageous in that it is noninvasive and applied ab externo. Unlike transscleral diathermy, it does not destroy or thin the sclera; unlike laser photocoagulation, it does not require a clear cornea to visualize the angle.[1]
Incisional Surgery
If more conservative measures fail, then incisional surgery may be attempted. Various techniques have been described. Three methods will be described in detail here.
The Direct Cyclopexy Technique[2]
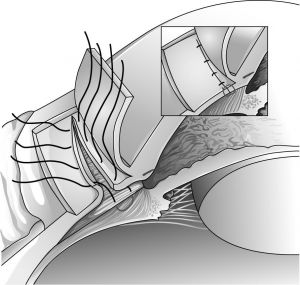
This method has the advantage of providing direct visualization of the disinserted ciliary body and scleral spur, allowing the most anatomically correct closure. It is more difficult and requires intimate knowledge of limbal anatomy. However, it is often the definitive procedure when other measures have failed.
- Anesthesia—Adequate anesthesia should be ensured with a retrobulbar, peribulbar, or sub-Tenon’s block.
- Anterior chamber formation—A paracentesis should be created and the anterior chamber filled with viscoelastic material to firm the globe and to allow better visualization of the cleft.
- Cleft identification—The location of the cleft is confirmed by gonioscopy under the microscope. A disposable cautery unit can be used to mark the extent and location of the cleft by making small burns at the limbus.
- Bridle suture—A bridle suture of braided polyglactin or polyester on a spatulated needle may be placed in the limbal cornea adjacent to the cyclodialysis cleft to allow rotation of the globe and adequate visualization.
- Conjunctival peritomy—This is typically performed with micro Westcott scissors. A small radial cut is made 3 to 4 mm beyond the edge of the cleft, 1 to 2 mm posterior to the limbus. Blunt dissection is used to remove Tenon’s membrane and conjunctiva from the globe. The scissor blades are kept parallel to the limbus and one blade is inserted into the sub-Tenon’s space. The blades are pulled gently toward the cornea and the conjunctiva is cut. This is repeated until the peritomy extends 3 to 4 mm beyond the opposite edge of the cleft.
- Cautery—Eraser cautery may be used to achieve hemostasis.
- Scleral flap—A two-thirds scleral thickness rectangular flap is created extending radially 4 mm from the limbus and 1 to 2 mm beyond the edge of the cleft in length. This may be done by using a crescent blade to create a two-thirds depth scleral groove 4 mm posterior to limbus and extending 1 to 2 mm beyond each edge of the cleft. A pocket blade may be used to create a scleral tunnel extending to the limbus. Vannas scissors may be used to cut at each side of the scleral tunnel, creating the scleral flap.
- Reattachment of the ciliary body—A full-thickness circumferential incision is made 1.5 mm posterior to the limbus within the scleral bed using a size 67 miniature surgical blade. This should result in entry into the cleft and release of aqueous. Direct visualization of the cleft should be made and light cautery applied to the exposed surface of the ciliary body. The ciliary body is then sutured to the sclera using interrupted 10-0 nylon sutures on a tapered vascular needle, spaced 1 mm apart. Each suture is passed first through the anterior lip of the scleral wound, exiting in the region of scleral spur. The next pass is through ciliary muscle away from the iris root vessels, and then through the posterior lip of the scleral flap. The sutures are then tied after all sutures have been placed (Figure 3).
- Cryotherapy—Light cryotherapy may be applied at the base of the scleral bed to promote adhesion.
- Flap closure—Simple interrupted 10-0 nylon sutures are placed at each corner of the scleral flap to suture the flap back into the clerval bed. Additional sutures should be placed as needed to securely close the flap.
- Conjunctival closure—Buried simple interrupted sutures of 8-0 polyglactin are placed at each edge of the peritomy to reap-proximate the conjunctiva to the limbus.
- Viscoelastic removal—Balanced salt solution on a cannula may be used to inject the solution into the anterior chamber and burp the viscoelastic out. Care should be taken to ensure that the paracentesis is watertight and the eye has a physiologic IOP when this is completed.
The Cross Chamber Cyclopexy Technique[2]
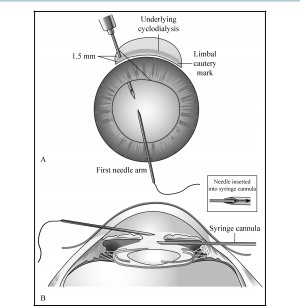
This is an indirect method of closure using techniques borrowed from suturing posterior chamber IOLs. It is less difficult than direct cyclo-pexy but may only be used in aphakic and pseudophakic eyes.
- Pupil dilation—Dilating drops should be applied preoperatively.
- Administration of anesthesia, anterior chamber formation with viscoelastic, cleft identification by gonioscopy, bridle suture placement, conjunctival peritomy creation, and cautery application are performed as described above.
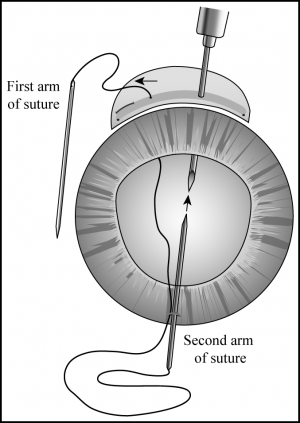
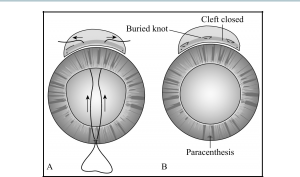
- Ciliary body reattachment—A 1- to 2-mm corneal keratotomy is created 180 degrees opposite the cleft with a sharp blade. A 27-gauge needle is passed ab externo through the sclera 1.5 mm posterior to the limbus at one end of the cleft, exiting through the ciliary sulcus between the iris and the posterior chamber lens. One arm of a 10-0 double-armed polypropylene suture is introduced through the keratotomy into the anterior chamber and is threaded deep into the barrel of the 27-gauge needle (Figure 4). The 27-gauge needle is pulled along with the straight needle out of the eye, leaving the polypropylene suture passing through the keratotomy, across the anterior chamber, through the ciliary sulcus, across the cleft, and out through the sclera. The 27-gauge needle is reinserted in the same fashion 3 mm adjacent to the previous entry site. The second arm of the suture is passed through the keratotomy and inserted into the barrel of the needle, then the needles are pulled out of the eye (Figure 5). The 2 ends of the suture should now both be lying outside the eye on the side with the cyclodialysis cleft. The needles are removed and the suture tightened and tied, pulling the ciliary body up against the scleral wall. This maneuver is repeated as often as needed to close the full length of the cleft (Figure 6). The suture knots should be buried.
- Cryotherapy—Light cryotherapy can be applied to the sclera posterior to the sutures to promote adhesion.
- Viscoelastic removal and conjunctival closure are performed as above.
Iris Base Fixation[2][17]
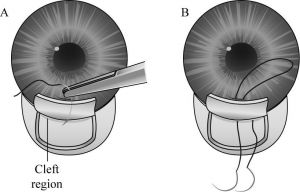
This is the easiest of the three incisional techniques but is only appropriate for smaller clefts of less than 2 clock hours because it creates broad peripheral anterior synechiae along the length of the cleft.
- Anesthesia administration, anterior chamber formation with viscoelastic, cleft identification, bridle suture placement, conjunc-tival peritomy creation, and cautery application are performed as described previously.
- Keratotomy—A 1- to 2-mm keratotomy is created through peripheral clear cornea 1 mm anterior to the limbus, adjacent to the cleft. For larger clefts, the keratotomy should be 1 mm shorter than the extent of the cleft.
- Scleral flap—A scleral flap is created as described previously.
- Ciliary body reattachment—One arm of a double-armed 10-0 nylon suture on a curved cutting needle is passed through the keratotomy, through peripheral iris at 1 end of the cleft, and then exits through the scleral bed 0.5 to 1 mm behind the limbus. Care should be taken not to snag the corneal endothelium. This maneuver is repeated with the second arm of the suture, catching iris 1 mm from the first iris pass (Figure 7). The suture is pulled taut to pull the peripheral iris against the scleral wall, then the suture is tied down. This often causes the pupil to peak somewhat toward the cleft. Additional mattress sutures are placed in the same fashion as needed to span the width of the cleft (Figure 8).
- Light cryotherapy at the base of the scleral flap, viscoelastic removal, scleral flap closure, and conjunctival closure are per-formed as noted previously.
Postoperative Care
Atropine 1% is used for 2 to 4 weeks, and steroids are avoided, if possible, after any of the previously mentioned surgical techniques. Post-operative topical antibiotics are also recommended after any of the previously described incisional surgical techniques. Sometimes the cleft does not immediately close, and a few weeks may pass before the hypotony is reversed, particularly after the nonincisional techniques.[12] However, after the cleft does close, an extreme spike in IOP and eye pain may occur, requiring treatment with topical and oral aqueous suppressants.[2] Patients should be warned of this possibility, and the surgeon may consider prescribing oral aqueous suppressants ahead of time to be used if the patient develops severe eye pain shortly after surgery. Pilocarpine can facilitate reopening of the cleft and thus should be avoided.[12]
Complications
Potential complications of any of the incisional surgical techniques include endophthalmitis and significant hemorrhage from the ciliary body. IOP elevation frequently accompanies successful cleft closure and can be dramatic. In eyes that have undergone direct cyclopexy, the pressure can rise high enough to dehisce the surgical wound if it has not been closed tightly. In most cases, the IOP can be controlled medically, and long-term treatment is usually not needed unless there has been pre-existing glaucoma.[1] One case series of cyclodialysis clefts repaired by direct cyclopexy found that the extent of the cyclodialysis cleft was positively correlated with the length of time needed for normalization of IOP, as well as with the accelerated development of cataract.[18]
Prognosis
Traumatic and inadvertent surgically induced cyclodialysis clefts seldom close spontaneously, especially more than 6 weeks after the inciting event. Spontaneous closure may occur more often in children.[17] The prognosis of cyclodialysis clefts after surgical intervention is quite good. Although delayed closure may result in permanently reduced visual acuity, some patients have been reported to improve even after prolonged periods of hypotony. In one case report, a patient treated with argon laser photocoagulation improved from 20/200 to 20/30 after 7 years of hypotony.[12][19] In a series of 58 eyes with hypotonous cyclodialysis clefts that had undergone intervention after a period of hypotony ranging from 3 weeks to 3.5 years, all patients without damage to the visual pathways had a postoperative visual acuity of 20/60 or better. However, intervening within 2 months resulted in better postoperative visual acuities of 20/20 to 20/25.[17] In contrast, a more recent series of 32 eyes showed no correlation between cleft duration and postoperative visual acuity, with durations ranging from 0.1 to 54 months.[18]
Argon laser photocoagulation and direct cyclopexy have each been shown to be highly successful in small case series. In a series of 9 patients with cyclodialysis clefts, 6 of 7 were successfully treated with argon laser photocoagulation, 2 with adjunctive diathermy, and 1 with adjunctive direct cyclopexy. In the same series, an eighth patient closed with conservative management and a ninth closed without treatment.[15] In another case series, the cyclodialysis clefts of 28 of 29 eyes were successfully closed with direct cyclopexy. This and another series demonstrated that 86% to 94% of patients experienced an improvement in visual acuity after direct cyclopexy. Mean postoperative visual acuities were 20/35 to 20/38.[10][18] Many other techniques have also been described as effective in case reports, including anterior scleral buckling, capsular tension rings, polymethyl methacrylate sulcus IOL implants, and gas endotamponade.[1]
Conclusion
Although conservative measures may result in closure of cyclodialysis clefts, laser, cryotherapy, or incisional surgical techniques are often required to close these clefts and reverse hypotony. Cryotherapy and laser photocoagulation may work well with smaller clefts, but incisional surgical techniques are generally needed to close larger clefts. These techniques include the direct cyclopexy technique, the cross-chamber cyclopexy technique, and iris base fixation.
Key Points
- Cyclodialysis clefts should be suspected in any eye with unexplained hypotony and a history of recent ocular surgery or trauma.
- Identification of cyclodialysis clefts may be assisted by the use of intra-cameral viscoelastic during gonioscopy, UBM, or anterior segment optical coherence tomography (AS-OCT).
- The size of the cleft does not correlate with the degree of hypotony.
- If conservative measures, including atropine 1% and cessation of steroids fail, then cryotherapy or argon laser should be attempted.
- If these methods also fail, then incisional surgical techniques should be used (eg the direct cyclopexy technique, the cross chamber cyclo-pexy technique, or iris base fixation technique).
- Postoperative care includes atropine 1% and avoidance of steroids.
- Patients should be warned that a dramatic IOP spike may occur immediately following successful closure of a cyclodialysis cleft. These IOP spikes should be treated with topical and oral aqueous suppressants.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Ioannidis AS, Barton K. Cyclodialysis cleft: causes and repair. Curr Opin Ophthalmol. 2010;21(2):150-154.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Leen MM, Mills RP. Low postoperative intraocular pressure. In: Spaeth G, ed. ophthalmic Surgery: Principles and Practice. Philadelphia, PA: Saunders; 2003:380-387.
- ↑ 3.0 3.1 Mushtaq B, Chiang MY, Kumar V, Ramanathan US, Shah P. Phacoemulsification, persistent hypotony, and cyclodialysis clefts. J Cataract Refract Surg. 2005;31(7):1428-1432.
- ↑ Esquenazi S. Management of a displaced angle-supported anterior chamber intraocular lens. Ophthalmic Surg Lasers Imaging. 2006;37(1):65-67.
- ↑ Aminlari A, Callahan CE. Medical, laser, and surgical management of inadvertent cyclodialysis cleft with hypotony. Arch Ophthalmol 2004;122(3):399-404.
- ↑ Viestenz A, Kuchle M. Retrospective analysis of 417 cases of contusion and rupture of the globe with frequent avoidable causes of trauma: the Erlangen Ocular Contusion-Registry (EOCR) 1985–1995 [German]. Klin Monbl Augenheilkd. 2001;218(10):662-629.
- ↑ Akimoto M, Tanihara H, Negi A, Nagata M. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol. 1994;112(12):1540-1544.
- ↑ Sihota R, Kumar S, Gupta V, et al. Early predictors of traumatic glaucoma after closed globe injury: trabecular pigmentation, widened angle recess, and higher baseline intraocular pressure. Arch Ophthalmol. 2008;126(7):921-926.
- ↑ Viikari K, Tuovinen E. On hypotony following cyclodialysis surgery. Acta Ophthalmol (Copenh). 1957;35(5):543-549.
- ↑ 10.0 10.1 Kuchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Report in 29 consecutive patients. Ophthalmology. 1995;102(2):322-333.
- ↑ Kuhl D, Mieler WF. Ciliary body. In: Kuhn F, Pieramici DJ, eds. Ocular Trauma: Principles and Practice. New York, NY: Thieme; 2002:157-168.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 Banta JT, Cebulla CM, Quinn CD. Closed globe injuries: anterior chamber. In: Banta JT, ed. Ocular Trauma. Philadelphia, PA: Saunders Elsevier; 2007:82-88.
- ↑ Simmons, ST. Basic and Clinical Science Course Section 10: Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2007.
- ↑ Mateo-Montoya A, Dreifuss S. Anterior segment optical coherence tomography as a diagnostic tool for cyclodialysis clefts. Arch Ophthalmol. 2009;127(1):109-110.
- ↑ 15.0 15.1 Ormerod LD, Baerveldt G, Sunalp MA, Riekhof FT. Management of the hypotonous cyclodialysis cleft. Ophthalmology. 1991;98(9):1384-1393.
- ↑ Krohn J. Cryotherapy in the treatment of cyclodialysis cleft induced hypotony. Acta Ophthalmol Scand. 1997;75(1):96-98.
- ↑ 17.0 17.1 17.2 Ormerod LD, Baerveldt G, Green RL. Cyclodialysis clefts: natural history, assessment, and management. In: Weinstein GW, ed. Open-Angle Glaucoma. New York, NY: Churchill Livingstone; 1986:201-225.
- ↑ 18.0 18.1 18.2 Hwang JM, Ahn K, Kim C, Park KA, Kee C. Ultrasonic biomicroscopic evaluation of cyclodialysis before and after direct cyclopexy. Arch Ophthalmol. 2008;126(9): 1222-1225.
- ↑ Delgado MF, Daniels S, Pascal S, Dickens CJ. Hypotony maculopathy: improvement of visual acuity after 7 years. Am J Ophthalmol. 2001;132(6):931-933.