Glaucoma Drainage Devices: Management of Intraoperative and Postoperative Complications
| Primary authors |
|
|---|
Intraoperative Complications
Tube Transection
Transection of a tube is more common during a revision than during initial insertion. If transaction happens at the time of initial insertion, it may be prudent to simply replace the device. However, the techniques described below can be used as well.
If transection occurs during revision or inadvertently during other ocular surgery, it may not be feasible to replace the device. Once transection has occurred, the eye will be very hypotonous, so consider placing viscoelastic into the anterior chamber. The transection itself can be repaired with a commercially available tube extender, the Model TE (New World Medical, Inc. Rancho Cucamonga, California). Although this is easy, the extender is somewhat bulky, needs to be covered with graft material, and may not be immediately available.

An alternative to an extender is to splice in a segment of 22-gauge angio-catheter (Figure 1). A short segment can easily slip over each cut end of the transected tube as a sleeve. A 9-0 Prolene suture can then be placed directly through the angiocatheter sleeve and cut tube segment end at each end to secure the sleeve.
See tube extension techniques here: https://www.youtube.com/watch?v=yLdI99T145k&t=10s
Tube Trimmed Too Short
If the plate cannot be moved forward enough, then the device can be replaced or the tube can be extended using one of 2 techniques described above.
Scleral Perforation
Scleral perforation is most likely to occur when the plate is being anchored to the sclera, but can occur during any scleral suture pass. Perforation may be recognized by vitreous presenting through the suture tract, pigment egress, and less commonly by hemorrhage or hypotony. Once recognized, the suture should be removed. If the needle may have passed through the retina, then cryotherapy should be applied over and surrounding the needle tract (typically 1 to 3 spots). The temperature is allowed to reach –40 degrees Celsius and remain there for approximately 5 to 10 seconds.
After applying cryotherapy, the surgery is resumed and the plate can be anchored in the same area (it may, in fact, provide a small scleral buckle effect if part of the plate is overlying the perforation). A cycloplegic should be instilled at the end of surgery, and a careful fundus examination performed as soon as possible. A vitreoretinal consultation may be prudent.
Leakage Through Sclerostomy Around the Tube
If there is not a good seal around the tube, then profound hypotony can result.[1] Minimal ooze will typically stop on its own in a few days. If you recognize more then slow ooze around the tube, the tube should be removed, the sclerostomy sutured tight with an 8 -0 Vicryl suture, and a new sclerostomy made. Occasionally it may work to tighten the existing sclerostomy with an 8-0 Vicryl suture, but serious hypotony can result if a good seal is not achieved.
Postoperative Complications
Although glaucoma tube shunts are subject to some unique complications, many are similar to those found in trabeculectomy. The reader is encouraged to review this chapter.
Hypotony
Assessment
Final intraocular pressure (IOP) after tube shunt placement is determined by the rate of aqueous production and the rate of aqueous egress through the tube and capsule. Egress is controlled by the thickness/permeability of the capsule surrounding the plate and the surface area of the plate. Hypotony is caused by an imbalance in which aqueous egress is relatively too high for the level of aqueous production.
In the early postoperative period, overfiltration is the most common cause of hypotony after tube implant surgery. In a valved device, overfiltration is usually due to a failure of the valve mechanism. In a nonvalved tube shunt, very early postoperative overfiltration may be due to inadequate occlusion of the tube, over-zealous fenestration of the tube, or leakage through the sclerostomy around the tube. Overfiltration occurring later than 5 to 6 weeks postoperatively is likely due to lack of adequate encapsulation of the plate.
Aqueous hyposecretion from active iridocyclitis, ischemia, cyclitic membranes, or other forms of ciliary atrophy can also lead to hypotony. Often, a combination of the above factors is at play (eg, an elderly white woman with poor healing and subnormal aqueous secretion develops intractable hypotony after implantation of a large, nonvalved plate).
As is the case after trabeculectomy, the decision of whether to intervene for hypotony must be made based on the present risk to vision. Uncomplicated hypotony can often be observed, wheras a flat anterior chamber needs immediate intervention.
Medical Management
Similar to trabeculectomy overfiltration, tube -shunt overfiltration will often respond to a reduction in steroid dose and, if necessary, cycloplegia. Tapering the steroids will facilitate plate encapsulation, a process than can take several weeks. Having the patient wear a shield at night and avoid eye rubbing can help prevent intermittent tube-corneal endothelial touch. Avoidance of the Valsalva maneuver should be recommended to reduce the risk of choroidal hemorrhage.
If hypotony is not resolving or if the anterior chamber is extremely shallow or flattened, viscoelastic can be injected into the anterior chamber to temporarily restrict flow through the tube. This can allow some time for the plate to encapsulate. In the setting of ciliary body detachment, which characteristically causes aqueous hyposecretion, the temporary rise in IOP provided the viscoelastic can allow reattachment of the ciliary body and improvement in aqueous secretion. Viscoelastic should be injected through a 27-gauge needle after sterilely prepping the eye and administering a topical antibiotic. Healon is a typical standard choice, and may be retained in the anterior chamber less than 24 hours. Healon GV and Healon 5 allow progressively longer retention and higher achievable IOP.
Surgical Management
If hypotony is not resolving spontaneously or if it is profound, surgical intervention is indicated. The easiest approach is to re-occlude the tube to give the plate more time to encapsulate. This may be done by making a small conjunctival cut-down just anterior to the plate and tightly occluding with a 7-0 Vicryl or 8-0 Prolene suture (the latter option should be wound twice around the tube). Alternatively, the tip of the tube can be delivered from the eye, occluded with an 8-0 Prolene suture wrapped twice, and replaced into the anterior chamber. (The occluding suture will be within the anterior chamber, allowing for easy laser access later.) Prolene sutures can then be “warmed” and loosened at a later date with an argon laser, 500 mW for 500 msec. A red laser may work better than a green laser for Prolene sutures.
If there is recalcitrant or late hypotony, the plate may simply be too large for the eye’s ability to produce aqueous and encapsulate the plate. The most assured “cure” of the hypotony is to remove the device (or cut off and remove the tube), although uncontrolled IOP elevation will often result. If the original device was a large, nonvalved plate, it may be replaced with a valved device. A perhaps less successful alternative is to replace it with a smaller version of the same device (eg, changing a Baerveldt 350 mm2 to a 250 mm2 or a Molteno3) or trimming the existing plate to a smaller size. Silicone or flexible plates can be easily cut to a smaller size with Stevens scissors.
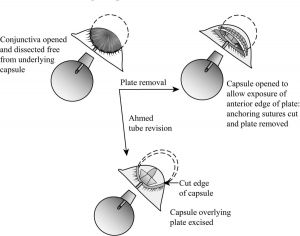
Removing a tube shunt begins by making a conjunctival incision 2 to 3 mm anterior to the plate, parallel to the plate, and at least as wide. The incision is then carried down through Tenon’s capsule to sclera and posteriorly to expose the anterior edge of the plate capsule. To help mobilize conjunctiva and eventual wound closure, blunt dissection is then used to separate conjunctiva from underlying capsule. A paracentesis is made so that the anterior chamber can be reformed as needed. The tube is then grasped near the plate (Figure 2) and pulled from the anterior chamber. Given the long tract under the conjunctiva and patch graft, there should not be much leakage, but the sclerostomy can be sutured as necessary by making a small peritomy at the limbus to elevate the patch graft. The capsule surrounding the plate should then be sharply entered near the anterior edge of the plate and the incision extended widely to expose the entire anterior edge of the plate. Next, cut the sutures anchoring the plate to the sclera. To find and cut the fibrous “stalks” that have grown through a Baerveldt’s plate fenestrations, place blunt-tipped scissors on top of the plate and “strum” the stalks to locate them. Most plates can now be easily pulled out, although large Baerveldt plates may be more easily removed if one wing is cut off first. If necessary, a smaller tube shunt can then be placed in the existing Tenon’s “pocket” or placed in a new quadrant.
Hypertensive Phase
Any type of glaucoma drainage device can go through a hypertensive phase, but it is more common, or at least more easily noticed, in valved devices that are not tied off initially. The hypertensive phase is typically seen at 4 to 5 weeks[2] postoperatively, when the IOP begins to rise. Often, a moderate to high encapsulated bleb is noted.
First-line treatment is to taper steroids if possible, because steroid-responsive IOP rise is definitely possible despite a functioning tube. Early use of aqueous suppressants can also be helpful, not only to lower the IOP but also to potentially “soften” the capsule by lessening internal pressure and stretching of the capsule. Typically, I will avoid early use outflow agents (ie, prostaglandin analogs) because they can be somewhat inflammatory in the early postoperative period and because there is already some functional outflow from the tube shunt.
Tube Exposure
Assessment
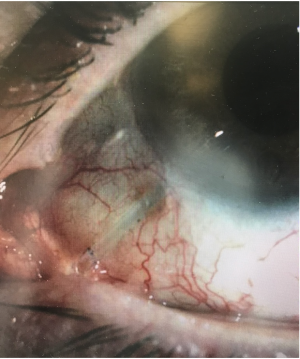
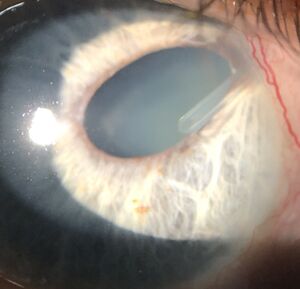
Erosion of the tissues overlying the tube (figure 3) usually occurs months to years after tube placement, although occasionally it can occur earlier. It is typically seen approximately 2 to 4 mm posterior to the limbus, presumably due to pressure and rubbing from the upper lid tarsus. Tube exposure needs to be repaired because it can lead to endophthalmitis and even orbital cellulitis. Although a patient may be temporized with topical antibiotics, management of tube exposures is purely surgical.
If signs of infection are seen, such as anterior chamber reaction or purulence, intense antibiotic treatment should be instituted and consideration given to tube removal. Clearly, any hint of endophthalmitis should be referred to a vitreoretinal specialist.
Surgical Management
In my experience, most cases of tube exposure have occurred in tubes originally covered by pericardium. If that is the case, I will try once to re-cover the tube with donor sclera or rarely split-thickness cornea, both of which are more durable in the eye than pericardium (if the tube erodes through sclera, I will often reposition the tube as described below). I typically make my incision at the limbus, anterior to the plate, or along the area of erosion depending on the location and amount of scarring. Careful, gentle dissection is then used to elevate conjunctiva off the tube and surrounding sclera to make a pocket large enough to accommodate a new graft. The new graft material is slipped into place and sutured with 8-0 Vicryl. Often, there is extensive scarring, and mobilizing adequate conjunctiva is difficult. Additionally, the original erosion leaves a hole in the conjunctiva that may overlie the new patch graft. Exposed scleral patch grafts will typically epithelialize and then vascularize in a few weeks. If a large area of patch graft remains exposed at the end of the surgery, amniotic membrane or a free conjunctival graft can be sutured to fill in the conjunctival defect.
If the tube erosion is recurrent or there is not enough mobile conjunctiva to allow re-covering of the tube, then the tube needs to be repositioned to change the way it interacts with the eyelid. One option is to remove the device (or just cut off and remove the tube) and place a new device in another quadrant. However, I have had excellent success by repositioning the tube into the pars plana. If the patient has not already had a complete pars plana vitrectomy with trimming of the vitreous skirt, I will perform the surgery in conjunction with one of my vitreoretinal colleagues. Once the vitreous has been cleared, I begin repositioning the tube by making a 2 to 3 clock-hour limbal peritomy with short radial relaxing incisions in the quadrant with the tube. Conjunctiva is elevated, the tube tip is removed from the eye, and the sclerostomy is sutured with 8-0 Vicryl. The tube is then placed into the pars plana through a fresh 23-gauge needle tract. For further details on pars plana placement of the tube, please see this chapter.
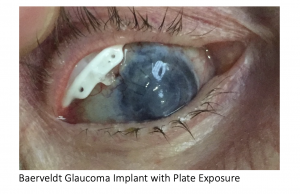
Plate Erosion
Late erosion of the plate (figure 4) is uncommon but cannot be repaired. Direct suturing of the conjunctiva or covering the plate with graft material almost always fails. In these cases, the exposed device should be removed. If necessary, another tube can be placed in a different quadrant, or ciliary body ablation can be performed.
Early postoperative wound dehiscence can be successfully resutured if there is healthy conjunctiva and minimal wound tension. Friable conjunctiva or excessive wound tension should be approached by replacing the device into another quadrant.
Tube Occlusion
Occlusion in the Anterior Chamber
Tube occlusion can occur at the tip of the tube in the anterior chamber. Occlusion is easily recognized in the anterior chamber if iris, fibrin/blood clot, silicone oil, or other substance is seen at the end of the tube in association with an elevated IOP.
Frequently, these occlusions may be cleared with the YAG laser. Rarely, pilocarpine helps pull iris out of the tube, but the iris may come back up into the tube at a later date. Fibrin or blood clot may be treated with tissue plasminogen activator (tPA), 3 to 25 μg injected into the anterior chamber through a 30-gauge needle. (An inpatient hospital pharmacy or compounding pharmacy can dilute the intravenous tPA solution to 100 to 250 microgram/mL, and 0.1 cc is injected at the slit lamp.[3]) Of course, intracameral tPA can lead to a total hyphema if fresh wounds or fragile vessels remain.
Occlusion of the Valve Mechanism
A valved device, in particular Ahmed tubes, can also become obstructed at the level of the valve mechanism. Obstruction can be due to material lodged in the valve mechanism or from fibrous bands growing retrograde from the plate up the tube. Valve obstruction is often difficult to differentiate from simple plate encapsulation. However, when the IOP is elevated, careful inspection of the bleb can be revealing. When the valve is obstructed, the bleb will be absent, the conjunctiva will be closely adherent to the plate, and detailed structures of the plate will frequently be visible through the conjunctiva. With plate encapsulation, the bleb may be low but not flat, and detailed structures of the plate are not visible. B-scan ultrasonography also may be helpful to look for fluid surrounding the plate.
If valve obstruction is confirmed, firm ocular massage may force aqueous through the valve and occasionally relieve the obstruction. This technique tends to work best in the first few days after surgery.
If massage fails, the tube may be considered to have failed. Options for managing tube failure are discussed below in the section on cyclodestruction.
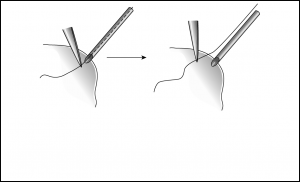
However, I have had success in surgically clearing a valve obstruction with a planned 2- step approach. In the operating room, begin by getting a careful baseline examination of the flat bleb because you will be looking for it to elevate when the obstruction is relieved. The first attempt to clear the obstruction is made by passing a 3-0 Prolene suture up the tube through the valve. The Prolene can be placed into the tube by delivering the tip of the tube from the eye, or by making a paracentesis 180 degrees away from the tube and running the tube across the anterior chamber. As the suture is advanced, you may feel resistance and then a “give” as the suture passes through the valve. Once the suture is inserted as far as it can go, it is important to ensure it has passed the valve (Figure 5), especially if it cannot be directly seen through the conjunctiva. With tying forceps, grasp the Prolene right at the tip of the tube (or at the paracentesis if the suture was passed through the cornea) and without letting go, pull the Prolene out. Your forceps now mark the maximum point where the suture was inserted, so you can lay the Prolene on the eye, lining up your forceps with the tube tip/paracentesis, to make sure adequate suture length was inserted to pass through the valve. Next, deepen the anterior chamber and raise the IOP substantially. Check to make sure that the bleb forms and that the IOP comes down over several minutes. Many times this maneuver will be successful.
If there is still no evidence of tube function, the obstruction is more likely due to fibrous growth into the valve or tight encapsulation overlying the valve outlet. I have found success in these situations by cutting away the capsule overlying the plate (only in valved devices, otherwise profound hypotony will Tube tip delivered from eye, 3-0 prolene run up tube and grasped with forceps to mark maximum point of insertion.
After suture is pulled from tube, it is lined up to ensure adequate length of suture was inserted to pass through valve result). Begin by making a conjunctival incision 2 to 3 mm anterior to, and parallel to, the plate and at least as wide as the plate. Carry it deep to the sclera, taking care to avoid cutting the tube. Identify the anterior edge of plate, and bluntly and broadly dissect conjunctiva and Tenon’s off of the capsule surrounding the plate. Next, sharply enter the capsule at the anterior edge of the plate and excise as much capsule tissue as possible. Often, as the thick encapsulation is pulled up off the plate, a fibrous extension can be seen extending into the valve. Once the valve is cleared, aqueous should flow immediately (if it does not, the plate should be replaced or the valve mechanism disassembled). Close the conjunctiva–Tenon’s complex with a running 8-0 Vicryl suture. To prevent the incision from overlying the plate where it can leak, take anterior anchoring bites of Tenon’s, scar, and/or sclera as the suture is run.
Tube Failure
For the purpose of this discussion, tube failure is considered to occur when the IOP remains too high despite a bleb over the plate and maximum tolerated hypotensive therapy.
This situation can be difficult to manage, and there are essentially 3 options: revision of the existing tube, placement of a second tube, or cyclodestruction.
Revision of the Existing Tube
This involves cutting away the thick capsule overlying the plate as described above. This can be successful in some cases, but the capsule may reform. In a valved device, this technique has the advantage of relatively rapid patient recovery and low operative risk. I will often try a revision of an Ahmed tube before placing a second device.
If a nonvalved device is revised, then it must be temporarily reoccluded to prevent profound hypotony. For that reason, I do not attempt to revise nonvalved devices.
Placement of a Second Tube Shunt
A second tube shunt is often quite successful, as long as there is an available quadrant to place it in. In most cases, I will implant a Baerveldt 250 mm2 or 350 mm2 as a second tube because it is low profile and highly effective. An Ahmed may be a reasonable second choice, but its higher profile can lead to proptosis or inferior globe diplacement when another drainage device is already in place. However, if the first implant provided very minimal benefit, then a second device may not work much better.
Cyclodestruction
Transscleral or endoscopic cyclophotocoagulation can be highly effective in the setting of some functional outflow (eg, the tube shunt). Treatment parameters are the same as for an eye without a tube shunt in place. For a transscleral approach, however, I will typically avoid treating the quadrant with the tube shunt because the graft and tube itself will make treatment of the underlying ciliary body less predictable. Cyclodestruction may be a less desirable in phakic patients, however, due to the rapid formation of advanced cataract.
Corneal Edema and Decompensation
The presence of any foreign object within the anterior chamber can lead to progressive loss of corneal endothelial cells and eventual corneal edema. The rate of endothelial cell loss has been reported at 18.6% at 24 months and even higher in the quadrant with the tube.[4]
Prevention
Progressive corneal endothelial cell loss is thought to be due to intermittent tube–corneal touch. A long tube or an anteriorly directed tube are risk factors for progressive corneal edema. Additionally, corneal endothelial cells may be lost at the site of the sclerostomy. Ideally, the sclerostomy should be made so that the tube enters posterior to Schwalbe’s line, which will ensure no portion of the tube is in contact with the corneal endothelium.
Surgical Management
If a tube is too long, the tip can be delivered from the anterior segment and trimmed. Alternatively, it can be trimmed directly within the anterior chamber.[5] If the tube is anteriorly directed, the tip should be removed, the sclerostomy sutured closed, and the tube reinserted in an appropriate direction.
When corneal edema related to a tube is noted, the tube should ideally be removed or repositioned into the posterior segment (please see this chapter). If visually significant corneal edema has already developed, a corneal endothelial graft or penetrating keratoplasty may be required. The success of a corneal graft in this situation may be improved if the tube is removed or repositioned posteriorly.
Iris Related Complications
Placement of a tube anterior or posterior to the iris can lead to various complications. It is possible to tear iris root or damage the body of the iris and related vascular structures during creation of the scleral entry with the 22 or 23 gauge needle. Proper visualization is always key to avoid trauma during this phase of surgery. It is also possible to place the tube in a position that causes pigment dispersion (more common with sulcus fixated tubes) as well as iritis due to constant rubbing against the iris body. Proper positioning over the surface of the iris (or deep to the posterior pigment aspect when placing in the sulcus) and away from the corneal endothelium is key to avoid these issues. Postoperatively, pupil distortion may occur due to the iris being displaced into the needle/tube entry point (Figure 6). While this can be well-tolerated, prompt attention is needed to remedy the situation, through surgical extraction of the iris and placing the patient on miotics for a brief course, prior to fibrosis of tissue precluding proper resolution.
Key Points
- A piece of 22-gauge angiocatheter can be spliced in to extend or repair a tube.
- Leakage around the tube can result in intractable hypotony, and anything more than a minimal ooze needs to be addressed before leaving the operating room.
- Early postoperative hypotony is likely due to failure of the valve mechanism is valved devices, poor occlusion or over-fenestration in nonvalved devices, or leakage around the tube in either type of device.
- Late postoperative hypotony is due to poor encapsulation of the plate and/or reduced aqueous production.
- A “hypertensive” phase is common, but typically IOP can be controlled by tapering steroids and adding ocular hypotensive medications.
- When the IOP is too high after implantation of a valved device, look for a flat bleb to rule out valve obstruction.
- Failed drainage devices can be addressed by revision (for valved devices), placing a second device, or cyclodestruction.
References
- ↑ García-Feijoó J, Cuiña-Sardiña R, Méndez-Fernández C, Castillo-Gómez A, García-Sánchez J. Peritubular filtration as a cause of severe hypotony after Ahmed valve implantation for glaucoma. Am J Ophthalmol. 2001;132(4):571-572.
- ↑ Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136(6):1001-1008.
- ↑ Zalta AH, Sweeney CP, Zalta AK, Kaufman AH. Intracameral tissue plasminogen activator use in a large series of eyes with valved glaucoma drainage implants. Arch Ophthalmol. 2002;120(11):1487-1493.
- ↑ Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148(3):361-367.
- ↑ Asrani S, Herndon L, Allingham RR. A newer technique for glaucoma tube trimming. Arch Ophthalmol. 2003;121(9):1324-1326.